Nimotop
Buy nimotop 30mg fast delivery
Local public health officials m ust develop partnerships w ithin the com m unity to assist w ith accom plishing these responsibilities muscle relaxant 500 mg purchase generic nimotop on line. Planners m ust select locations, establish facility use agreem ents, and coordinate staffing and security plans. Som e jurisdictions purchase m edication caches for first responder com m unities for im m ediate prophylaxis during a large-scale inhalation anthrax attack or pandem ic influenza. Planners m ay find that they face additional planning considerations associated w ith such caches, such as political guidance, cost, storage, and regulatory issues that influence this decision. State and local planners should w ork together to determ ine how m uch m edication is needed and how m uch can feasibly be stored and m aintained in state or local caches. To leverage buying pow er partner agencies and organizations m ay decide to establish an agreem ent to m ake a collective purchase versus m ultiple sm aller purchases. A single storage and m aintenance agreem ent also m ight m ake the m anagem ent of procured counterm easures a cost savings for the com m unity. However, to dispense m edications in this condensed tim efram e, the jurisdiction m ay need to alter and/or elim inate standard dispensing procedures in order to save tim e and in turn, save lives. Chapter 8: Dispensing M edical Counterm easures provides further inform ation on legal and regulatory considerations. State dispensing laws It is im portant for planners to be aw are of the law s that govern w ho can legally dispense m edications w ithin the state. In addition, planners need to be fam iliar w ith law s governing w ho can issue standing orders for m edication dispensing and w hether that authority is 16 ftp://ftp. Planners should w ork with their public health law professionals to determ ine the needs of their jurisdictions. Staff Com pensation Plans should include inform ation regarding w hether and how the jurisdiction w ill com pensate staff and volunteers for their efforts during a public health response. Planners should w ork w ith their public health law professionals to determ ine how com pensation w ill occur and clearly include the com pensation m echanism s in the plan. In addition, planners should docum ent any m em oranda of agreem ent (M O A) or m em oranda of understanding (M O U) w ith entities that w ill participate w ithin their jurisdiction during a public health response and those M O As or M O Us should clearly define w hether and how the jurisdiction w ill provide com pensation. This includes em ergency response providers as defined in section 2 of the Hom eland Security Act of 2002 (6 U. Em ergency response providers are federal, state, and local em ergency public safety, law enforcem ent, em ergency response, em ergency m edical (including hospital em ergency facilities), and related personnel. As broad as these definitions read, it is im perative for jurisdictions to clearly identify w ho qualifies as a first responder w ithin their com m unities and w hat protective 19 w w w. Based on lessons learned from the response to Hurricane Katrina in 2005, health officials m ay also w ant to consider planning for first responders to have access to prophylactic m edications for their fam ily m em bers to help ensure that these essential groups of w orkers are able to respond w ithout fam ily-related reservations. Critical infrastructure includes assets, system s, and netw orks, w hether physical or virtual. These system s are vital to com m unities and their incapacitation or destruction w ould have a debilitating effect on security, the econom y, public health, safety, or any com bination thereof. M ost com m only recognized critical infrastructure assets are facilities and system s for. W ater supply (drinking w ater, w aste w ater/sew age, stem m ing of surface w ater);. Continuity planning facilitates the perform ance of essential functions during an em ergency that disrupts norm al operations and/or the tim ely resum ption of norm al operations once the em ergency has ended. A strong continuity plan provides the organization or com m unity w ith the m eans to address the num erous issues involved in perform ing essential functions and services during an em ergency. W ithout detailed and coordinated continuity plans and effective continuity program s to im plem ent these plans, jurisdictions risk leaving their residents w ithout vital services in w hat could be their tim e of greatest need. Achieve the tim ely and orderly resum ption of essential functions and the return to norm al operations;. M eet the operational requirem ents of the respective organization (continuity plans m ay need to be operational w ithin m inutes of activation, depending on the essential function or service, but certainly should be operational no later than 12-hours after activation);. M eet the sustainm ent needs of the respective organization (An organization m ay need to plan for sustained continuity operations for up to 30 days or longer, depending on resources, support relationships, and the respective continuity strategy adopted);. Ensure the continuous perform ance of essential functions and operations during an em ergency, including such instances as pandem ic influenza that m ay require additional considerations beyond traditional continuity planning; and. Provide an integrated and coordinated continuity fram ew ork that takes into consideration other relevant organizational, governm ental, and private sector continuity plans and procedures. M aintaining and Reviewing the Plan It is im portant to note that plans should adhere to a regular cycle of updates. Every update of the plan should include a review, evaluation, and approval by responsible agencies. Portions of the plan m ight be shared am ong agencies, so planners should consider how to protect any confidential inform ation in the plan. Regardless of w hat inform ation is shared, each agency involved w ith executing the plan needs to have a copy of the portion that pertains to their particular responsibilities. Planners m ust ensure thorough dissem ination, coordination of resources, execution of agreem ents, volunteer coordination, training, exercising, and plan im provem ent and updating. O nce a form al plan is developed and w ritten, jurisdictions should have regularly scheduled review s of the w ritten plan to update inform ation, add new partners, and include changes gleaned from exercises and after action reports. Ideally, each jurisdiction should review and update their plan at least annually and share any pertinent changes w ith neighboring jurisdictions and response partners. See Chapter 13: Training, Exercising, and Evaluating Plans for further inform ation on exercising, evaluating, and updating the plan based on after action item s from drills and exercises. All team m em bers, as w ell as alternate or back-up team m em bers, should be fam iliar w ith their particular roles and responsibilities during a response. It is im portant to ensure these roles are filled by individuals w ho both possess skills appropriate for the position and are available to fill the position during a response. Planners should look to the agencies and organizations that participate in the m ultidisciplinary advisory group and associated partner agencies listed in Table 2. Team m em bers selected from know ledgeable personnel within the jurisdiction bring skills and expertise that can be incorporated into the overall construction and execution of the plan. For instance, all areas have law enforcem ent agencies that are fam iliar w ith security and tactical com m unication system s and m any have a transportation departm ent that is fam iliar w ith dispatching and tracking vehicles for distribution efforts. The staffing and volunteer coordinator m ust have strong know ledge of volunteer recruitm ent and coordination, the ability to m anage and assign tasks to staff from a variety of disciplines, fam iliarity w ith dispensing law s and regulations in the jurisdiction, and the ability to determ ine staffing needs for the jurisdiction, be it the state, local, or dispensing level. Determ ine credentialing procedures and licensing requirem ents for m edical volunteers. Possible sources for staffing and volunteer coordinators include health departm ent personnel, em ergency m anagem ent agencies, and volunteer and com m unity service organizations. Fortunately, states have em ergency com m unication system s that allow for diversity and redundancy. Regardless of w hether the backbone of the com m unications system is a law enforcem ent agency, state em ergency m anagem ent, or a departm ent of transportation, any com m unication system m ust have the capability to transfer inform ation from all levels and all players in the response com m unity. To lead com m unication efforts, the jurisdiction w ill need som eone w ho is fam iliar w ith the prim ary com m unication system and has a w ide background and know ledge in other system s. This position is a technical position and is not a public inform ation specialist. To ensure these actions take place, the strategic and tactical com m unications coordinator m ust develop detailed com m unication netw orks and support plans. Chapter 10: Strategic and Tactical Com m unications provides additional inform ation that can assist in determ ining the lead for this position. Security Coordinator Security provides a safe environm ent for response activities and the person w ho fills this role should possess a law enforcem ent background. Security leads should have a strong know ledge of facility and personnel security, law enforcem ent security policies and procedures, traffic m anagem ent, and personnel identity and badging procedures. Jurisdictions m ay identify security team leads from state or local law enforcem ent professionals, private security professionals, or the National G uard. Jurisdictions m ay find it advantageous to partner w ith or contract professional w arehouse m anagers to fill this position. Inventory M anagem ent Lead the lead for inventory m anagem ent activities provides inventory status, including supply, resupply, allocations, and possible shortages to incident com m and during an incident.
Diseases
- Progressive myositis ossificans
- Chromosome 14, trisomy mosaic
- Mesomelic dwarfism cleft palate camptodactyly
- Lopez Hernandez syndrome
- PIRA
- L?ri Weill dyschondrosteosis
- Xerostomia
- Opsismodysplasia
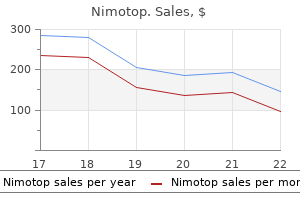
Cheap generic nimotop canada
Meningococcal conjugate vaccine can be given to a pregnant woman when there is increased risk of disease muscle relaxant mechanism discount nimotop 30mg without a prescription, such as during epidemics or before travel to an area with hyperendemic infection. Infection with hepatitis A or hepatitis B can result in severe disease in a pregnant woman and, in the case of hepatitis B, chronic infection in the newborn infant. Hepatitis A or hepatitis B immunizations, if indicated, can be given to pregnant women. Initiation of the vaccine series should be delayed until after completion of the pregnancy. If a woman is determined to be pregnant after initiating the immunization series, the remainder of the 3-dose regimen should be delayed until after completion of the pregnancy. If a vaccine dose has been administered during pregnancy, no intervention is needed. Rabies vaccine should be given to pregnant women after exposure to rabies under the same circumstances as nonpregnant women. No association between rabies immunization and adverse fetal outcomes has been reported. If the risk of exposure to rabies is substantial, preexposure prophylaxis also may be indicated. Anthrax vaccine is inactivated and has no theoretical risk to the fetus, but the vaccine has not been evaluated for safety in pregnant women, so it should be avoided unless in a postevent situation with a high risk of exposure (see Anthrax, p 234). Primary disorders of the immune system generally are inherited, usually as single-gene disorders; can involve any part of the immune defenses, including B-lymphocyte (humoral) immunity, T-lymphocyte (cell)-mediated immunity, complement and phagocytic function, and innate immunity; 2 and share the common feature of increased susceptibility to infection. In general, people who are severely immunocompromised or in whom immune function is uncertain should not receive live vaccines, either viral or bacterial, because of the risk of disease caused by the vaccine strains. Additional vaccines not given universally are indicated for children with certain conditions. Immunocompromised patients should avoid contact with people who develop skin lesions after receipt of varicella or zoster vaccines until lesions clear. Inactivated vaccine administration can be deferred temporarily until corticosteroids are discontinued if the hiatus is expected to be brief and adherence to return appointment is likely. Application of low-potency topical corticosteroids to localized areas on the skin; administration by aerosolization; application on conjunctiva; or intraarticular, bursal, or tendon injections of corticosteroids usually do not result in immunosuppression that would contraindicate administration of live-virus vaccines. Children who are receiving only maintenance physiologic doses of corticosteroids can receive live-virus vaccines. Some experts, however, would delay immunization with live-virus vaccines until 2 weeks after discontinuation. These children should not be given live-virus vaccines, except in special circumstances. These agents often are used in combination with other immunosuppressive drugs, such as methotrexate or corticosteroids. Vaccination status should be assessed pretreatment, and recommended vaccines should be administered with timing as for planned corticosteroid use (see Timing of Vaccines). Patients 12 months or older who have not received hepatitis A vaccine (HepA), did not complete the vaccination series, or who are seronegative should receive the HepA vaccine series. Household members of these patients should be counseled about risks of infection and should have vaccination status made current. All infants, children, adolescents, and adults with asplenia, regardless of the reason for the asplenic state, have an increased risk of fulminant septicemia, especially associated with encapsulated bacteria, which is associated with a high mortality rate. In comparison with immunocompetent children who have not undergone splenectomy, the incidence of and mortality rate from septicemia are increased in children who have had splenectomy after trauma and in children with sickle cell disease by as much as 350-fold, and the rate may be even higher in children who have had splenectomy for thalassemia. The risk of invasive bacterial infection is higher in younger children than in older children, and the risk may be greater during the years immediately after surgical splenectomy. Fulminant septicemia, however, has been reported in adults as long as 25 years after splenectomy. Streptococcus pneumoniae is the most common pathogen causing septicemia in children with asplenia. Those with functional or anatomic asplenia also are at increased risk of fatal malaria and severe babesiosis. Immunization Pneumococcal conjugate and polysaccharide vaccines are vital for all children with asplenia (see Pneumococcal Infections, p 626). A second dose should be administered 5 years later (see Pneumococcal Infections, p 626). Previously unimmunized children with asplenia younger than 5 years should receive Hib vaccine according to the catch-up schedule. Unimmunized children 5 years or older should receive a single dose of Hib vaccine. When surgical splenectomy is planned, immunization status for Hib, pneumococcus, and meningococcus should be ascertained, and all indicated vaccines should be administered at least 2 weeks before surgery. If splenectomy is emergent, or vaccination was not performed before splenectomy, indicated vaccines should be initiated as soon as possible 2 weeks or more after surgery. Management options include postponement of splenectomy for as long as possible in people with congenital hemolytic anemia, preservation of accessory spleens, performance of partial splenectomy for benign tumors of the spleen, conservative (nonoperative) management of splenic trauma, or when feasible, repair rather than removal. Antimicrobial Agents Daily antimicrobial prophylaxis against pneumococcal infections is recommended for many children with asplenia, regardless of immunization status. Less agreement exists about the need for prophylaxis for children who have had splenectomy after trauma. In general, antimicrobial prophylaxis (in addition to immunization) should be considered for all children with asplenia younger than 5 years of age and for at least 1 year after splenectomy at any age. On the basis of a multicenter study, prophylactic penicillin can be discontinued at 5 years of age in children with sickle cell disease who are receiving regular medical attention, are fully immunized, and have not had a severe pneumococcal infection or surgical splenectomy. The appropriate duration of prophylaxis for children with asplenia attributable to other causes is unknown. Some experts continue prophylaxis throughout childhood and into adulthood for particularly high-risk patients with asplenia. For antimicrobial prophylaxis, oral penicillin V (125 mg, twice a day, for children younger than 3 years; and 250 mg, twice a day, for children 3 years or older) is recommended. For children with anaphylactic allergy to penicillin, erythromycin can be given (250 mg, twice daily). A substantial percentage of pneumococcal isolates have intermediateor high-level resistance to penicillin, resistance to macrolides and azalides, or both. When antimicrobial prophylaxis is used, these limitations must be stressed to parents and patients, who should be made aware that all febrile illnesses potentially are serious in children with asplenia and that immediate medical attention should be sought because the initial signs and symptoms of fulminant septicemia can be subtle. Likewise, medical attention should be sought for asplenic patients who suffer animal bites. In some clinical situations, other antimicrobial agents, such as aminoglycosides, may be indicated. Parenteral antimicrobial therapy also is given in the perioperative period for cochlear implantation and reparative neurosurgical procedures. Infants and children with a personal or family history of seizures of any etiology might be at greater risk of having a febrile seizure after receipt of one of these vaccines compared with children without such histories. No evidence indicates that febrile seizures cause permanent brain damage or epilepsy, aggravate neurologic disorders, or affect the prognosis for children with underlying disorders. In the case of pertussis immunization during infancy, vaccine administration could coincide with or hasten the recognition of a disorder associated with seizures, such as infantile spasms or severe myoclonic epilepsy of infancy, which could cause confusion about the role of pertussis immunization. Hence, pertussis immunization in infants with a history of recent seizures generally should be deferred until a progressive neurologic disorder is excluded or the cause of the earlier seizure has been determined. The nature of seizures and related neurologic status are more likely to have been established in children by the age of 12 months. Postimmunization seizures in these children are uncommon, and if they occur usually are febrile in origin, have a benign outcome, and are not likely to be confused with manifestations of a previously unrecognized neurologic disorder. Chronic diseases may make children more susceptible to the severe manifestations and complications of common infections. However, live-virus vaccines are contraindicated in children with severe immunologic disorders, including children receiving chronic immunosuppressive therapy (see Immunocompromised Children, p 74). All children with chronic liver disease are at risk of severe clinical manifestations of acute infection with hepatitis viruses and should receive hepatitis A (HepA) and hepatitis B (HepB) vaccines on a catch-up schedule if they have not received vaccines routinely (see Hepatitis A, p 391, and Hepatitis B, p 400). Siblings of children with chronic diseases and children in households of adults with chronic diseases should receive recommended vaccines, including both live and inactivated vaccines ( Immunization in American Indian/Alaska Native Children Although indigenous populations worldwide have high morbidity and mortality from infectious diseases, including vaccine-preventable infections, all indigenous populations are not the same (nc.
Order nimotop 30 mg without prescription
Recrudescence of P falciparum and P malariae infection occurs when a persistent low-concentration parasitemia causes recurrence of symptoms of the disease or when drug resistance prevents elimination of the parasite spasms youtube purchase 30 mg nimotop visa. In areas of Africa and Asia with hyperendemic infection, repeated infection in people with partial immunity results in a high prevalence of asymptomatic parasitemia. The spread of chloroquine-resistant P falciparum strains throughout the world is of increasing concern. In addition, resistance to other antimalarial drugs also is occurring in many areas where the drugs are used widely. If initial blood smears test negative for Plasmodium species but malaria remains a possibility, the smear should be repeated every 12 to 24 hours during a 72-hour period. It is the only antigen-detection kit available and is approved for use by hospitals and commercial laboratories. Also, information about the sensitivity of rapid diagnostic tests for the 2 less common species of malaria, P ovale and P malariae, is limited. Effective measures to reduce the risk of acquiring malaria include control of Anopheles mosquito populations, protection against mosquito bites, treatment of infected people, and chemoprophylaxis of travelers to areas with endemic infection. Drugs used for malaria chemoprophylaxis generally are well tolerated, although adverse reactions can occur. Travelers with serious adverse reactions should be advised to contact their physician. Notice to readers: new medication for severe malaria available under an investigational new drug protocol. If there is desire to ensure tolerance of the antimalarial drug to be used for prophylaxis, then the drug should be started earlier so that there is time to assess any adverse events before departure and time to change to another effective drug if needed. Adverse reactions that can occur include gastrointestinal tract disturbance, headache, dizziness, blurred vision, insomnia, and pruritus, but these generally are mild and do not require discontinuation of the drug. Atovaquone-proguanil is taken daily, starting 1 day before exposure and continuing for the duration of exposure and for 1 week after departure from the area with endemic malaria. A pediatric formulation is available in the United States but is not approved for prophylaxis in children weighing less than 11 kg. The rare adverse effects reported by people using atovaquoneproguanil for chemoprophylaxis are abdominal pain, nausea, vomiting, mouth ulcers, and headache. Travelers taking doxycycline should be advised of the need for strict adherence to daily dosing; the advisability of always taking the drug on a full stomach; and the possible adverse effects, including diarrhea, photosensitivity, and increased risk of monilial vaginitis. Parents should be advised not to travel to countries with endemic malaria with children weighing less than 5 kg or younger than 6 weeks because of the risks associated with infection (septicemia or malaria) in young infants. Malaria may increase the risk of adverse outcomes in pregnancy, including abortion, preterm birth, and stillbirth. Harmful effects on the fetus have not been demonstrated when chloroquine is given in the recommended doses for malaria prophylaxis. Pregnancy and lactation, therefore, are not contraindications for malaria prophylaxis with chloroquine. Travelers to malaria-endemic settings should seek medical attention immediately if they develop fever. Malaria can be treated with good results if begun early in the course of disease, but delay of appropriate treatment can have serious or even fatal consequences. Travelers who do not take an antimalarial drug for prophylaxis, who are on a less-than-effective regimen, or who may be in very remote areas can be given a reliable supply of atovaquone-proguanil or artemether-lumefantrine. If they are diagnosed with malaria while traveling, they will have a medicine that will not interact with their other medications, is of good quality, and is not depleting local resources. Travelers taking atovaquone-proguanil as their chemoprophylactic drug regimen should not take atovaquone-proguanil for treatment and should use an alternative antimalarial regimen recommended by a travel medicine expert. Rarely, travelers exposed to primaquine-resistant or -tolerant parasites may require high-dose primaquine. All travelers to areas where malaria is endemic should be advised to use personal protective measures, including the following: (1) using insecticideimpregnated mosquito nets while sleeping; (2) remaining in well-screened areas; (3) wearing protective clothing; and (4) using mosquito repellents. To be effective, most repellents require frequent reapplications (see Prevention of Mosquitoborne Infections, p 213). Acute encephalitis, which often results in permanent brain damage, occurs in approximately 1 of every 1000 cases. In the postelimination era, death, predominantly resulting from respiratory and neurologic complications, has occurred in 1 to 3 of every 1000 cases reported in the United States. Measles is transmitted by direct contact with infectious droplets or, less commonly, by airborne spread. In temperate areas, the peak incidence of infection usually occurs during late winter and spring. In the prevaccine era, most cases of measles in the United States occurred in preschooland young schoolaged children, and few people remained susceptible by 20 years of age. Resuming progress toward 2015 milestones and elimination goals will require countries and their partners to raise the visibility of measles elimination, address barriers to measles vaccination, and make substantial and sustained additional investments in strengthening health systems. Vaccine failure occurs in as many as 5% of people who have received a single dose of vaccine at 12 months or older. Although waning immunity after immunization may be a factor in some cases, most cases of measles in previously immunized children seem to occur in people in whom response to the vaccine was inadequate (ie, primary vaccine failures). This was the main reason a 2-dose vaccine schedule was recommended routinely for children and high-risk adults. Patients are contagious from 4 days before the rash to 4 days after appearance of the rash. Immunocompromised patients who may have prolonged excretion of the virus in respiratory tract secretions can be contagious for the duration of the illness. Isolation of measles virus is not recommended routinely, although viral isolates are important for molecular epidemiologic surveillance. The sensitivity of measles IgM assays varies by timing of specimen collection, immunization status of the case, and the assay. Measles IgM is detectable for at least 1 month after rash onset in unimmunized people but might be absent or present only transiently in people immunized with 1 or 2 vaccine doses. Therefore, a negative IgM test result should not be used to rule out the diagnosis in immunized people. In populations with high vaccine coverage, such as the United States, it is recommended that diagnostic testing for measles include both serologic and virologic testing. Vitamin A treatment of children with measles in developing countries has been associated with decreased morbidity and mortality rates. Parenteral and oral formulations of vitamin A are available in the United States. Measles vaccine should be considered in all exposed individuals who are vaccine-eligible and who have not been vaccinated or have received only 1 dose of vaccine. If the exposure does not result in infection, the vaccine should induce protection against subsequent measles exposures. Immunization is the intervention of choice for control of measles outbreaks in schools and child care centers and for vaccine-eligible people 12 months and older. The only measles vaccine licensed in the United States is a live further-attenuated strain prepared in chicken embryo cell culture. Measles-containing vaccines can be given simultaneously with other immunizations in a separate syringe at a separate site (see Simultaneous Administration of Multiple Vaccines, p 35). A small proportion (5% or less) of immunized people may lose protection after several years. The second dose provides protection to those failing to respond to their primary measles immunization and, therefore, is not a booster dose. Prevention of varicella: update of recommendations for use of quadrivalent and monovalent varicella vaccines in children. The period of risk for febrile seizures is from 5 to 12 days following receipt of the vaccine. People traveling internationally (any location outside of the United States) should be immune to measles. There is no evidence that reimmunization increases the risk of adverse events in people already immune to these diseases.
Purchase 30 mg nimotop with amex
Suppress rat populations by wellplanned and energetic campaigns of poisoning and with vigorous concurrent measures to reduce rat harbourages and food sources muscle relaxant half-life purchase nimotop 30mg without prescription. All are highly effective if used early (within 8?18 hours after onset of pneumonic plague). After a satisfactory response to drug treatment, reappearance of fever may result from a secondary infection or a suppurative bubo that may require incision and drainage. Alert existing medical facilities to report cases immediately and to use full diagnostic and therapeutic services. Antibiotic prophylaxis should be undertaken for those with close documented exposure (see 9B5). On arrival of an infested or suspected infested ship, or an infested aircraft, travellers may be disinsected and kept under surveillance for a period of not more than 6 days from the date of arrival. Immunization against plague cannot be required as a condition of admission to a territory. For these reasons, a biological attack with plague is considered to be of serious public health concern. In some countries, a few sporadic cases may be missed or not attributed to a deliberate act. Any suspect case of pneumonic plague should be reported immediately to the local health department. The sudden appearance of many patients presenting with fever, cough, a fulminant course and high case-fatality rate should provide a suspect alert for anthrax or plague; if cough is primarily accompanied by hemoptysis, this presentation favors the tentative diagnosis of pneumonic plague. Depending on the extent of dissemination, mass prophylaxis of potentially exposed populations may be considered. In Europe and North America, pneumococcal pneumonia is estimated to affect approximately 100 per 100 000 adults each year. Clinical manifestations typically include sudden onset, high fever (with shaking chill or rigor and/or other systemic symptoms like myalgia, arthralgia, headache, malaise), pleural pain, dyspnoea, tachypnoea and cough productive of rusty sputum. The onset may be less abrupt, especially in the elderly, and fever, shortness of breath or altered mental status may provide? In infants and young children, fever, vomiting and convulsions may be the initial manifestations. Typical chest X-ray shows lobar or segmental consolidation; consolidation may be bronchopneumonic, especially in children and the aged. The case-fatality rate, formerly 20%?40% among hospitalized patients, has fallen to 5%?10% with antimicrobial therapy, but remains 20%?40% among patients with substantial underlying disease or alcoholism (it may exceed 50% in the high-risk groups). In developing countries the case-fatality rates in children are often over 10% and as high as 60% in infants under 6 months. Secondary pneumococcal pneumonia is often observed in the vulnerable population and among previously healthy individuals, following other respiratory infections. The presence in sputum of many Gram-positive diplococci together with polymorphonuclear leukocytes suggests the diagnosis, which can be con? For severe cases suspected to have bacterial pneumonia, treatment should not be delayed and empiricial antimicrobial therapy should start before microbiological con? It is important to identify the etiological agent together with its antimicrobial susceptibility. Infectious agent?Streptococcus pneumoniae (pneumococcus), a Gram-positive encapsulated coccus often colonizing the human nasopharynx, where it can be carried asymptomatically. Current data suggest that the 11 most common serotypes cause at least 75% of invasive disease in all regions. Occurrence?A disease of continuing endemicity, particularly in infancy and old age and in individuals with underlying medical conditions; more frequent in malnourished populations, the lower socioeconomic groups and in developing countries. It occurs in all climates and seasons, incidence being highest in winter and spring in temperate zones. Recurring epidemics have been described among South African miners; incidence is high in certain geographic areas. High-level antibiotic resistance to essential anti-microbials such as penicillin, cephalosporins and macrolides is a serious and rapidly increasing problem worldwide. Pneumococci are commonly found in the upper respiratory tract of healthy people worldwide. Mode of transmission?Droplet spread, direct oral contact, or indirectly through articles freshly soiled with respiratory discharges. Person-to-person transmission of the organisms is common, but illness among casual contacts and attendants is infrequent. Period of communicability?Presumably until discharges of mouth and nose no longer contain virulent pneumococci in signi? Penicillin will render patients with susceptible strains noninfectious within 24?48 hours. Susceptibility?Susceptibility is general and disease may occur in persons susceptible to the serotype involved. Susceptibility to symptomatic pneumococcal infection is increased by processes affecting the integrity of the lower respiratory tract, including in? Malnutrition and low birthweight are important risk cofactors for pneumonia among infants and young children in developing countries. In February 2000 a new 7-valent conjugate vaccine, which apparently reduces invasive disease by about 70%, was approved for use in children even under 2. For most eligible patients, vaccine need be given only once; however, reimmunization is generally safe, and vaccine should be offered to eligible patients whose immunization status cannot be determined. Reimmunization is recommended once for persons over 2 who are at highest risk for serious pneumococcal infection. Reimmunization after 3 years should also be considered for children with functional or anatomic asplenia and those who present conditions associated with rapid antibody decline after initial immunization. In addition, persons 65 and older should be given another dose of vaccine if they received the vaccine more than 5 years previously and were under 65 at the time of primary immunization. Most of the pneumococcal antigen types in the vaccine are poor immunogens in children under 2. This has been effective in preventing pneumococcal pneumonia and meningitis in young children and infants. Control of patient, contacts and the immediate environment: 1) Report to local health authority: Obligatory report of epidemics in some countries; no individual case report, Class 4 (see Reporting). Penicillin G, parenterally, is the preferred treatment (erythromycin for those hypersensitive to penicillin). For pneumonia and other pneumococcal infections, parenteral beta-lactam antibiotics are likely to be effective in most cases. Where beta-lactam resistance is common, vancomycin should be included in initial regimens for the treatment of meningitis possibly due to pneumococci until susceptibilities can be determined (in some counties use of vancomycin is limited because of concern for adverse effects). Epidemic measures: In outbreaks in institutions or in other closed groups, immunization may be carried out unless it is known that the type causing disease is not included in the vaccine. Disaster implications: Crowding of populations in temporary shelters bears a risk of disease, especially for the very young and the elderly. Onset is gradual with headache, malaise, cough (often paroxysmal), sore throat and sometimes chest discomfort that may be pleuritic. In severe cases, the pneumonia may progress from one lobe to another and become bilateral. Diagnosis is based on a rise in antibody titres between acute and convalescent sera; titres rise after several weeks. Infectious agent?Mycoplasma pneumoniae belongs to the Mycoplasmas (Molicutes), placed between bacteria and viruses. Mycoplasmas lack cell walls, cell wall synthesis inhibitors such as the penicillins and cephalosporines are therefore not effective in treatment. Attack rates vary from 5 to more than 50/1000/year in military populations and 1 to 3/1000/year in civilians. Epidemics occur more often in late summer and autumn; endemic disease is not seasonal, but there can be variation from year to year and among different geographic areas. The disease is asymptomatic or mild in children under 5; recognized disease is most frequent among school-age children and young adults. Mode of transmission?Probably droplet inhalation, direct contact with an infected person (probably including those with subclinical infections) or with articles freshly soiled with nose and throat discharges from an acutely ill and coughing patient. Secondary cases of pneumonia among contacts, family members and attendants are frequent. Treatment does not eradicate the organism from the respiratory tract, where it may persist for as long as 13 weeks. Disease varies from mild afebrile pharyngitis to febrile illness of the upper or lower respiratory tract.
Deanol hemisuccinate (Deanol). Nimotop.
- Unwanted movements of the face and mouth (tardive dyskinesia).
- What is Deanol?
- How does Deanol work?
- Dosing considerations for Deanol.
- Improving exercise performance (when used with ginseng, vitamins, and minerals).
- Are there safety concerns?
- Any other medical condition, including attention deficit-hyperactivity disorder (ADHD), aging skin, declining memory and mood, improving intelligence and physical energy, preventing aging or liver spots, improving red blood cell function, improving muscle reflexes, increasing oxygen efficiency, extending life span, and treating autism.
- Are there any interactions with medications?
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96526
Order nimotop cheap
Cosmetic botulinum toxin treatments are not Other rare complications associated with botulicovered by insurance muscle relaxant chlorzoxazone buy nimotop 30mg free shipping. Fees for injections range from $250 num toxin injections include formation of antibodies to $550 per treatment area and are based on the number (less than 1%), which can render treatments ineffecof botulinum toxin units used or on the area treated. Food and Drug of response and patient-reported outcomes after onabotulinumtoxinA Administration; September 11, 2013. A multicenter, double-blind, randomized, placebo-controlled Lippincott Williams & Wilson; 2009:200-213. Botulinum toxin: examining duration of effect in facial aesment of glabellar lines. Complications, adverse reactions, and insights with the use of 2003;29(5):461-467. Managing adverse events associated with botulidomized comparison of 3 doses of botulinum toxin type A and placebo num toxin type A: a focus on cosmetic procedures. Safety of botulinum toxin type A: a systematic ized comparison of onabotulinumtoxinA (Botox) and abobotulinumreview and meta-analysis. Botox cosmetic (onabotulinumtoxinA) for injection, for intramuscular 2010;24(11):1278-1284. ConsenabobotulinumtoxinA (marketed as Dysport) and rimabotulinumtoxinB sus recommendations on the use of botulinum toxin type a in facial (marketed as Myobloc). Triple-blind, prospective, internally conof botulinum toxin: case report and review of the literature [in Chinese]. Dermatologic and Cosmetic Procedures in cosmetic injections with an unlicensed, highly concentrated botulinum Offce Practice. Inhalational Botulism in Rhesus M acaques Exposed to Botulinum Neurotoxin Complex Serotypes A1 and B1 1 1 1 1 2 Daniel C. During the course of this study, clinical observations, body weights, clinical hematology results, clinical chemistry results, circulating neurotoxin levels, and telemetric parameters were documented to aid in the understanding of disease progression. Clinical observations were consistent with the recognized pattern of botulism disease progression. Dose-related changes in physiologic parameters measured by telemetry were also observed. In contrast, notable changes in body weight, hematology, and clinical chemistry parameters were not observed. Army Medical Research Institute for Infectious potential threat and have listed them as a category A threat, a Diseases (5), are identi? A licensed vaccine is not currently requires the development and use of relevant animal models. Animal studies under the animal rule require a thorough understanding of the pathophysiological mechanism of the pathogenic agent and demon* Corresponding author. E-mail: challenge agent used for animal studies should be identical to jshearer2@csc. The mixture (for each mouth breathing, serous nasal discharge, salivation, dysphavial) was injected i. Western blotting was also performed to tant for maintaining consistency among studies. Protein concentration and biological activity (in terms different production facilities varies. Performance characteristics of the head-only aerosol exposure system were established prior to challenging animals. General procedures for animal care and housing were in accordance determine the total volume of air that the animal needed to inspire to achieve a with the Guide for the Care and Use of Laboratory Animals (17), and studies were targeted exposure dosage. Exposure duration was based on the time required for conducted under protocols approved by the Battelle Laboratory Animal Care each animal to achieve the designated volume of inspired air measured by and Use Committee. The estimated inhaled dose was calculated using the this study (three extra monkeys were available for replacement purposes). All monkeys Each stage consisted of a prechallenge period (minimum of 7 days) and a had three consecutive negative tuberculin tests (performed during their quaranpostchallenge period, which extended to day 30. The mixture was then injected intraperichallenged with four target challenge doses ranging from 37. At the conclusion of each stage, mortality results mortality was demonstrated in the test sample group. All monkeys tested negawere analyzed and target exposure doses for the next stage determined. The following tissues were collected during persistent prostration, or total paralysis), or signs of pneumonia. All monkeys necropsy, preserved, and processed for histopathological evaluation: adrenal meeting any of the above criteria or judged to be terminally ill by the veterinary glands, aorta, bone marrow (sternum), brain, esophagus, eyes, heart, large instaff were euthanized with an overdose of a euthanasia agent containing pentotestine (cecum, colon, and rectum), kidneys, liver, lungs with main-stem bronchi barbital. Monkeys were weighed prior to challenge on study day 0 and at teric), mammary glands, optic nerves, ovaries (females), pancreas, pituitary, least every 3 days until death/euthanasia or until the end of the postchallenge prostate (males), mandibular salivary glands, sciatic nerve, skeletal muscle, small period (day 30). Blood samples were collected from unlumbar), spleen, stomach, testes with epididymides (male), trachea, thymus, anesthetized animals from a femoral artery or vein prior to challenge on study thyroid and parathyroid, urinary bladder, and uterus with cervix (females). These tissues were processed also collected prior to euthanasia of moribund animals. The pleura pressure catheter was implanted beneath the serosal layer of calculated in this way represents the aerosol exposure concentration that, if the esophagus within the thoracic cavity. Transmitters (surgically implanted in monkeys), receivers were assigned to the high-dose group. Overall, body weights collected for monistry or hematology parameter, and for each study day, before and after outliers were excluded. Baseline-adjusted hourly postchallenge values were from the corresponding monkeys prechallenge. First, values averaged hourly for 24 h were calculated (over Clinical observations. The most common clinical observa7 days) for the prechallenge baseline data for each animal. Then, daily hourly tions postchallenge were anorexia, lethargy, ataxia, labored averages (24 h per day for 30 study days or until death) for the postchallenge data were calculated for each animal. Finally, for each animal, the baseline-adjusted respiration, dysphagia, ptosis, nasal discharge, paresis, coughhourly postchallenge value at each postchallenge hour was calculated by subing, mouth breathing, and lateral recumbency. Baseline data analysis was perand in this group anorexia was observed more frequently than formed on the values averaged hourly for 24 h for the seven telemetry paramin low-dose-challenged animals. Additional abnormal clinical using the dose group and survival status (low-dose animals, middle-dose animals observations (other than anorexia) were documented in midthat survived, middle-dose animals that died, and high-dose animals) as a single dle-dose animals that died, including labored respiration, lethmain effect. For the 8-hour models, random animal and repeated-measurement effects were taken into account in the calculation. Table S2 in the supplemental material based on inhaled dosage per kg of body weight and the expoprovides a complete listing of the numbers and percentages of sure concentrations are shown in Table 2. In this monkey, lethargy, umented approximately 72 h postchallenge, with all three monataxia, coughing, nasal discharge, and dysphagia were? Other telemetry parameters (activity, pulse pressure from baseline early, at 152 h postchallenge. The pulse presTherefore, subsequent statistical analyses were performed only sure for these middle-dose survivors returned to nonsigni? The upper shaded section of each panel shows clinical observations in middle-dose-group monkeys that survived. The middle, more lightly shaded section of each panel shows clinical observations in middle-dose-group monkeys that died. The bottom, unshaded section of each panel shows clinical observations of monkeys in the high-dose group. For other signs, the duration of clinical signs is displayed by a line segment beginning at the time at which the sign was? Anorexia was a common sign observed in all dose groups and was the only abnormal sign observed in low-dose-group animals. Anorexia was noted intermittently throughout the postchallenge period and varied from monkey to monkey.
Buy nimotop paypal
The spore pellet was resuspended in distilled water and lyophilized spasms after eating order cheap nimotop online, and scraped into glass vials for Raman spectral measurements. Approximately 1 gram each, determ ined to be 99% pure by microscopic observation, was produced for this study. The vial was never moved from the sample holder to ensure that the same portion of silver-doped sol-gel was examined. Throughout this process, no more than 20 drops of acid or base were added, and therefore the concentration was diluted by no more than 10%. Initial spore experiments employed 78 oC, 50 mM dodecylamine to digest the spores and release dipicolinic acid for measurement. Final spore measurements employed room temperature acetic acid to digest the spores. Pre-weighed spores were used to prepare a stock solution for calibration using a counting grid (see below), from which a known number of spores were dried on a glass plate. The alkoxide and silver amine precursors were mixed in an 8/1 v/v ratio, then 140 L were introduced into 2 mL glass vials, which were then spin-coated. L of the mixed precursors were then drawn into a 1-mm diameter glass capillary coating a 15-mm length. After sol-gel formation, the incorporated silver ions were again reduced with dilute sodium borohydride. An f/2 achromat was used to collimate laser beam exiting the source fiber optic, while a 4 mm prism was used to direct the beam through an f/0. The scattered radiation was collected back along the same optical axis, while a second f/2 lens focused the beam into the collection fiber optic. A short pass filter was placed in the excitation beam path to block the silicon Raman scattering generated in the source fiber from reflecting off sampling optics and reaching the detector. A long pass filter was placed in the collection beam path to block the sample Rayleigh scattering from reaching the detector. For 785 nm excitation, a similar optic probe was used, Detection of Bacillus Spores by Surface-Enhanced Raman Spectroscopy 23 except a dichroic filter was used to reflect the laser light to sample and pass the Raman scattered radiation to the collection fiber. In the case of Raman spectral measurements of spores, the samples were placed on a glass slide with the probe aimed downward. Relative standard deviations for all concentrations are reported as percent standard deviation in Table 2. Results and discussion the present application begins with a Raman spectral analysis of Bacillus spores with regards to contributions from calcium dipicolinate. Spectral conditions: 500 mW of 1064 nm at the sample, 5 min acquisition time, 8 cm-1 resolution. Since this amount will be used to calculate the number of spores measured, it is important to have as accurate a number as possible. In particular, the 1014 cm-1 peak noticeably changes intensity, especially when compared to the neighboring phenylalanine peak at 1003 cm-1. If it can be assumed that the composition of these Bacilli is very similar, then it may be assumed that the relative phenylalanine concentration is nearly constant and its Raman peak can be used as an internal intensity standard. In the latter case, a recent study using resonance Raman spectroscopy of the same sample Fig. It is more likely that experimental conditions during the original growth of the bacteria, such as time, temperature, and available nutrients, influenced the extent of sporulation. However, it is usually beneficial to acquire and examine both when making assignments. Notably, the 760 cm-1 peak in the solid phase is completely absent in the solution phase, while a new peak at 1386 cm-1 appears in the solution phase. Detection of Bacillus Spores by Surface-Enhanced Raman Spectroscopy 27 peak is likely associated with carboxylic acid groups. Spectral conditions: A) 450 mW of 785 nm, 5 min acquisition time and B) 150 mW of 785 nm, 1-minute acquisition time; both 8 cm-1 resolution. These species may interact with the silver quite differently and consequently influence the amount that each vibrational mode is enhanced. Furthermore, added enhancement might be expected for the vibrational modes of the deprotonated carboxylic acid groups that participate in this interaction, or for modes that are favorably aligned perpendicular to the surface due to this interaction. Overall there is only a modest decrease in intensity for most of the peaks as a function of pH. For example, the 1006 cm-1 peak assigned to the pyridine ring stretching mode decreases by ~7% from pH 2 to 11. The greatest changes observed, yet still modest, are in the peak intensities at 795, 812, 1567, and 1590 cm-1 between pH 1. The 795 cm-1 peak loses intensity as the pH becomes basic, while the 812 cm-1 peak gains a little intensity. Similarly, the 1567 cm-1 peak loses intensity as the pH becomes basic, while the 1590 cm-1 peak gains intensity. The peak heights were divided by the peak height of the 1006 cm-1 peak at each pH and then scaled with the lowest value set to 0 and the highest to 1 g/L. A) the 1006 cm-1 peak intensity is shown as measured, but scaled to a 0 to 1 g/L concentration range. B) the 795 and 812 cm-1 peak intensities are normalized to the 1006 cm-1 peak intensity and then scaled. Spectral conditions: 100 mW of 785 nm, 44 sec acquisition time, 8 cm-1 resolution. However, a 35 cm-1 shift is somewhat inconsistent with a weak analyte-to-surface interaction. This shift may 30 Bioterrorism be due to the silver surface influencing the carboxylic acid dissociation energy. A preliminary calibration curve was prepared by measuring 100, 50, 20, 10, 5, 2, 1, 0. Spectra were measured at nine points along the length of each capillary and the median values are plotted in Fig. It is obvious that the response is not linear, in that the peak heights change from 0. The signal was taken as the height of the 1006 cm-1 peak, while the noise was the relative standard deviation of baseline noise measured between 50 and 150 cm-1. This is consistent with the fact that attempted measurements of 1 g/L samples did yield spectra, but not in every case. In both cases, 1-mm capillaries were used to hold the samples, and the same sample optics were used. Taking the concentration into account yields an estimated enhancement factor of 2. It is difficult to determine the precise number of molecules in the field of view for the sol-gel, and this number may represent better than average enhancement, i. Recent estimates suggest that this mass corresponds to 1000 spores (Inglesby et al. Specifically, a 2 mg sample was placed in 2 mL of 5 mM dodecylamine in ethanol that was heated and maintained at 78 oC for 40 minutes. First, the sensitivity is insufficient, and second the use of a hot reagent severely limits its practical use in the field. To overcome these limitations we investigated numerous acids, bases and solvents. In fact the 1006 cm-1 peak intensity, once corrected for the difference in laser power is nearly identical for the same concentration in water (compare 0. Detection of Bacillus Spores by Surface-Enhanced Raman Spectroscopy 35 concentration in acetic acid, in which the dominant 1006 cm-1 peak intensity is held constant and the base line noise is observed to increase with decreasing concentration. As a specific example, a 2 mg sample of spores was weighed and dispersed in 10 mL of water by vortexing. The spores per volume water were calibrated by placing 10 L on a standard hemocytometer counting grid. Microscope images of 10 grids were recorded and 870 spores were counted, which represents ~22,000 spores per L (Fig. The original spore solution was diluted by a factor of 10 to produce a 2200 spore per L. From this solution, a 1 L sample was placed on a glass slide and allowed to dry producing a 2200 spores per ~0. Although this represents 11,000 spores/cm2, 3-orders of magnitude greater than the target sensitivity, no attempt was made to spread the spores across the surface.
Buy nimotop discount
Each lot of master seed virus should be tested for immunogenicity before use in manufacture of vaccine muscle relaxant and anti inflammatory buy genuine nimotop line. To test for susceptibility draw blood samples from each lamb and test the collected serum with a virus neutralisation test using a constant virus/varying serum method. Five replicate virus titrations should be conducted on a sample of the vaccine to be used. Take and record the rectal temperature for 17 consecutive days beginning 3 days pre-challenge. The master seed virus lot should be retested for immunogenicity at least once each 3 years using 5 vaccinated and 5 control susceptible lambs. In-process control All ingredients of animal origin, including serum, primary cells or cell lines, must be tested for presence of viable bacteria, viruses, fungi, or mycoplasmas according to the same protocol recommended for testing master seed virus. If cell lines are used they should be examined for characteristics determined to be normal to the cell line. Batch control a) Sterility Each batch (serial) of vaccine should be tested for presence of viable bacteria, extraneous virus, fungi, or mycoplasmas according to the same protocol recommended for testing master seed virus. To conduct the mouse safety test 8 adult mice should be inoculated intracerebrally with 0. If unfavourable reactions attributable to the product occur in 2 or more mice in either group the batch must be considered unsatisfactory and discarded. If unfavourable reactions occur in 2 or more mice which are not attributable to the vaccine the test is inconclusive and must be repeated. To conduct the sheep safety test inject 2 lambs at least 6 months of age with the equivalent of 10 doses of the vaccine and observe the sheep for 21 days. If unfavourable reactions not attributable to the vaccine occur the test is inconclusive and must be repeated. The vaccine should maintain a titre of 10 greater than that used for the immunogenicity test until the end of the expiration period. The development of a modified live virus vaccine employing American strains of bluetongue virus. Bluetongue virus: Comparative evaluation of enzyme-linked immunosorbent assay, immuno diffusion, and serum neutralisation for detection of viral antibodies. A clinical diagnosis may often be made on the basis of the generalised skin eruptions. A confirmatory laboratory diagnosis is required, particularly when differentiation from parapox virus infection is necessary. Identification of the agent: Full skin thickness biopsies should be taken for virus isolation, preferably within one week of the first appearance of the lesions. The tissue should be homogenised and ultrasonicated, or frozen and thawed to release intracellular virus. Cell cultures prepared from lamb testis, goat testis or kidney may be used to culture the virus. Characteristic intracytoplasmic pox virus inclusions become evident within a few days of inoculation. However, differentiation from lumpy skin disease is not possible by serological methods. Differentiation from contagious pustular dermatitis, caused by a parapox virus, may be made by immunodiffusion tests, or by electron microscopy, when the parapox and capripox virions are readily distinguishable. The size of the capripox virus is 300-400 nm, whereas that of parapox is approximately 200-250 nm. Serological tests: Indirect fluorescent antibody, agar gel immunodiffusion and virus neutralisation tests have been used most frequently for the investigation of capripox virus infections. Requirements for biological products: There is a wide range of live virus vaccines and inactivated vaccines for the prophylaxis of these diseases. A diagnosis may be based on the appearance and distribution of the generalised skin eruptions that occur on affected animals. Lamb kidney, calf kidney or testis, and ovine or bovine fetal muscle skin, lung or other cells can also be used. Tissue samples from skin biopsies of early lesions, or from lesions in the lung, may be used for virus isolation. They are minced with sterile scissors and then ground in sterile sand with a pestle and mortar. The mixture is left at 25?C for one hour and then ultrasonicated, or frozen and thawed 3 times using dry ice and alcohol. Virus particles, identifiable as capripox virus, may be seen by electron microscopy of this tissue suspension. This becomes more obvious and involves most of the monolayer after a further 4-9 days. Identification of viral antigens may be made by haematoxylin and eosin staining of coverslips to show the eosinophilic intracytoplasmic inclusions that are characteristic of pox viruses. The coverslips are washed, air dried and fixed in cold methanol for 10 minutes, then stained by direct or indirect immunofluorescence methods. A direct conjugate can be made from antisera of hyperimmunised rabbits or from experimentally infected sheep or goats. Coverslips infected with material from the suspected case, together with positive and negative controls, are stained and examined. It must be remembered that there are considerable cross-reactions between members of the pox virus group in agar gel immunodiffusion tests. Anti-sheep or anti-goat immunoglobulin conjugates can be obtained commercially or prepared in the laboratory. Positive sera have titres up to 1:500 to 1:5,000 in the 2-3 months following infection. The constant virus-varying serum method is recommended, using a serum dilution range of 1:5 to 1:500, although higher neutralising titres may be obtained. This has been overcome by using cultures of the less sensitive fetal muscle cells. Serological and cross-immunity tests show that most, if not all, strains of sheepor goat-adapted capripox viruses will cross-immunise. Characteristics Virus strains used for production must be adequately characterised as to their pathogenicity, immunogenicity, and freedom from adventitious virus or other agents. Their pathogenicity must be adequately tested in the breeds of animal which are to be vaccinated. A good deal of variation in the susceptibility of different breeds to the same virus strains can be found. Identification of the virus is carried out by the inoculation of cultures and examining them by direct immunofluorescence. Uninoculated control cultures should be maintained, and examined for non-cytopathogenic viral agents such as the Pestiviruses, Border disease, and bovine viral diarrhoea. Only scrapie-free flocks should be used for the preparation of primary cell cultures. Manufacture Monolayers prepared in stationary or roller bottles should be used, the latter being rolled at approximately 6-8 revolutions per hour. Ultrasonication, or freezing and thawing, is necessary to release cell-associated virus. A sugar and protein stabiliser may be incorporated during lyophilisation, for which sucrose, lactose, peptone, and lactalbumin have all proved satisfactory. Inactivated vaccines are prepared from cell cultures infected with a pathogenic virus strain of low passage history. An equal volume of alhydrogel is added to the virus-saline mixture and stirred for 48 hours at 4?C. In-process control Inoculated and uninoculated tissue culture vessels should be observed for any signs of nonspecific degeneration of the cell monolayer. Pooling of individual harvests may be delayed until all are shown to be free from bacterial contamination by overnight incubation in a suitable broth culture. Sheep pox and goat pox (A10) 89 b) Safety tests the vaccine is inoculated subcutaneously into 4 sheep or goats. They are examined daily for any febrile reaction, clinical signs or other untoward reaction. Titration of the virus in cell cultures, or the intradermal inoculation of sheep or goats is used to determine vaccine potency.
Discount 30mg nimotop fast delivery
Staff members at the scene of an injury or bleeding incident who do not have access to gloves need to use some type of barrier to avoid exposure to blood or blood-containing materials muscle relaxant 751 buy discount nimotop on-line, use appropriate hand hygiene measures, and adhere to proper protocols for handling contaminated material, including feminine hygiene products. Parents and students should be educated about the types of exposure that present a risk for school contacts. This may be protective for other participants and for infected athletes themselves, decreasing their possible exposure to bloodborne pathogens other than the one(s) with which they are infected. Wrestling and boxing probably have the greatest potential for contamination of injured 3 skin by blood. Athletes should be told not to share personal items, such as razors, toothbrushes, and nail clippers, that might be contaminated with blood. Even if these precautions are adopted, the risk that a participant or staff member may become infected with a bloodborne pathogen in the athletic setting will not be eliminated entirely. Caregivers should cover their own damaged skin to prevent transmission of infection to or from an injured athlete. Wounds must be covered with an occlusive dressing that will remain intact and not become soaked through during further play before athletes return to competition. The decontaminated equipment or area should be in contact with the bleach solution for at least 30 seconds. The area then may be wiped with a disposable cloth after the minimum contact time or allowed to air dry. Guidelines for prevention of intravascular catheter-related 1 infections also are available. Physicians and infection-control professionals should be familiar with this increasingly complex array of guidelines, regulations, and standards. To accomplish this goal, infection-control programs run by pediatric infectious diseases specialists increasingly are used in hospital settings; to be sustainable over time, these programs require adequate institutional support. Ongoing infection-prevention and -control programs should include education, implemention, reinforcement, documentation, and evaluation of recommendations on a regular basis. Isolation Precautions Isolation precautions are designed to protect hospitalized children, health care personnel, and visitors by limiting transmission of potential pathogens within the health care setting. Adherence to these isolation policies, supplemented by health care facility policies and procedures for other aspects of infection and environmental control and occupational health, should result in reduced transmission and safer patient care. Adaptations should be made according to the conditions and populations served by each facility. Hand hygiene should be performed either with alcohol-based agents or soap and water before donning and immediately after removing gloves, between patient contacts, and when otherwise indicated to avoid transfer of microorganisms to other patients and to items in the environment. Hand hygiene also should be performed after removal of gloves, even if visible soiling did not occur. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings 2007. For patient protection, use of a mask by the person inserting an epidural anesthesia needle or performing myelograms when prolonged exposure of the puncture site is likely to occur. Soiled patient-care Handle in a manner that prevents transfer of microorganisms to equipment others and to the environment; wear gloves if visibly contaminated; perform hand hygiene after contact with soiled items and after glove removal. Environmental control Develop procedures for routine care, cleaning, and disinfection of environmental surfaces, especially frequently touched surfaces in patient care areas. Used textiles (linens) and Handle in a manner that prevents transfer of microorganisms to laundry others and the environment. Injection practices (use of Do not recap, bend, break, or hand manipulate used needles; if needles and other sharps) recapping is required, use a one-handed scoop technique only; use needle-free safety devices when available; place used sharps in conveniently placed, puncture-resistant container. Use a sterile, single-use, disposable needle and syringe for each injection given. Single-dose medication vials are preferred when medications are administered to more than one patient. Patient resuscitation Use mouthpiece, resuscitation bag, or other ventilation devices to prevent contact with mouth and oral secretions. Patient placement Prioritize for single-patient room if patient is at increased risk of transmission, is likely to contaminate the environment, does not maintain appropriate hygiene, or is at increased risk of acquiring infection or developing adverse outcome following infection. Recommendations for Application of Standard Precautions for Care of All Patients in All Health Care Settings, continued Component Recommendations Respiratory hygiene/cough Instruct symptomatic people to cover mouth/nose when sneezing/ etiquette (source concoughing; use tissues and dispose in no-touch receptacle; obtainment of infectious serve hand hygiene after soiling of hands with respiratory tract respiratory tract secretions secretions; wear surgical mask if tolerated or maintain spatial in symptomatic patients) separation more than 3 feet, if possible. To prevent needlestick injuries, safety devices should be used whenever they are available. Needles should not be recapped, purposely bent or broken by hand, removed from disposable syringes, or otherwise manipulated by hand. After use, disposable syringes and needles, scalpel blades, and other sharp items should be placed in puncture-resistant containers for disposal; puncture-resistant containers should be located as close as practical to the use area. Large-bore reusable needles should be placed in a puncture-resistant container located close to the site of use for transport to the reprocessing area to ensure maximal patient safety. Sharp devices with safety features are preferred whenever such devices have equivalent function to conventional sharp devices. The 3 types of transmission routes on which these precautions are based are: airborne, droplet, and contact. Microorganisms transmitted by the airborne route can be dispersed widely by air currents and can be inhaled by a susceptible host within the same room or a long distance from the source patient, depending on environmental factors. Special air handling and ventilation are required to prevent airborne transmission. If susceptible people must enter the room of a patient with measles or varicella infection or an immunocompromised patient with local or disseminated zoster infection, a mask or a respiratory protective device (eg, 6! Because these relatively large droplets do not remain suspended in air, special air handling and ventilation are not required to prevent droplet transmission. Spatial separation of more than 3 to 6 feet should be maintained between the bed of the infected patient and the beds of the other patients in multiple bed rooms. Direct contact transmission involves a direct body surface-to-body surface contact and physical transfer of microorganisms between a person with infection or colonization and a susceptible host, such as occurs when a health care professional examines a patient, turns a patient, gives a patient a bath, or performs other patient care activities that require direct personal contact. Direct contact transmission also can occur between 2 patients when one serves as the source of the infectious microorganisms and the other serves as a susceptible host. Indirect contact transmission involves contact of a susceptible host with a contaminated intermediate object, usually inanimate, such as contaminated instruments, needles, dressings, toys, or contaminated hands that are not cleansed or gloves that are not changed between patients. If unavailable, cohorting patients likely to be infected with the same organism and use of Standard and Contact Precautions are permissible. Cohorting of children infected with the same pathogen is acceptable if a single-patient room is not available, a distance of more than 3 feet between patients can be maintained, and precautions are observed between all contacts with different patients in the room. If gloves are worn for diaper changing?for example, for women who are pregnant or likely to be pregnant, and in cases in which soiling of hands is likely?hand hygiene should be performed before and after the diaper changing. Single-patient rooms are recommended for all patients for TransmissionBased Precautions (ie, Airborne, Droplet, and Contact). Because most young children are incontinent, this recommendation does not apply to routine care of uninfected children. These recommendations do not apply to schools, out-of-home child care centers, and other settings in which healthy children congregate in shared space, including ambulatory care settings. The occurrence of these preventable infections is viewed as a patient safety issue, and there has been an increased emphasis on prevention. Most studies documenting a favorable effect of implementation of infection-prevention bundles have been performed in adult populations, and studies of infection-prevention strategies in pediatric patients are limited. Best-practice bundles in pediatrics have been developed to target reducing central line-associated bloodstream infections and ventilator-associated pneumonias. Educate health care personnel in central venous catheter insertion and maintenance techniques relevant to infection prevention, typically with a course or video. Although chlorhexidine is not approved for use in children younger than 2 months because of absence of safety data, a growing number of institutions are using it routinely on neonates and young infants; use of chlorhexidine in preterm infants is controversial. For neonates weighing less than 1500 g at birth, an iodine-based antiseptic is recommended. Occupational Health Transmission of infectious agents within health care settings is facilitated by close contact between patients and health care personnel and by lack of hygienic practices by infants and young children. People with commonly occurring infections, such as gastroenteritis, dermatitis, herpes simplex virus lesions on exposed skin, or upper respiratory tract infections, should be evaluated to determine the resulting risk of transmission to patients or to other health care personnel. Health care personnel education, including understanding of hospital policies, is of paramount importance in infection control.

Order nimotop 30mg online
Food sources and products causing outbreaks Until the early 1960s nearly all outbreaks of botulism in which toxin types were determined were caused by type A or B toxins and were usually associated with ingestion of home-canned vegetables muscle relaxer 86 67 purchase generic nimotop pills, fruits, and meat products. Type E botulism was not recognized as a (13,14) major problem in the United States until 1963, when 22 cases were reported. Sixty-one of the 67 outbreaks of type E botulism reported from 1950 through 1996 have been traced to marine products (fish or marine mammals); several cases have been attributed to beaver. Only three outbreaks of Type F botulism have been reported in (14-16) this country with one being traced to home-prepared venison jerky. Vegetables were the most important vehicle for the botulism toxin in the Untied States from 1950 through 1996. Fish and marine mammals were also responsible for a large number of the botulism outbreaks during this period. Of the 87 outbreaks caused by marine products (fish, or marine mammals), 61 were due to type E, 15 to type A, 8 to type B, and 3 were unknown. Prevention and control In the United States, foodborne botulism due to commercial foods has been largely controlled by safe canning and food manufacturing processes. Commercial canned foods are heated to a sufficient temperature and for a sufficient time to kill the spores. Unheated commercial foods in cans or jars can be made safe by acidification or other manipulations that inhibit the growth of the organism. Occasionally, commercial foods still cause botulism if they are prepared in a way that permits toxin production. Many outbreaks of foodborne botulism in the United States result from eating improperly (18) preserved home-canned foods. Persons doing home canning and other food preservation should be educated about the proper time, pressure, and temperature required to destroy spores, the need for adequately refrigerated storage of incompletely processed foods, and the effectiveness of (19) boiling, with stirring, home-canned vegetables to destroy botulinum toxins. Therefore, heating home-canned foods before consumption can reduce the risk of botulism intoxication. Infant botulism Since 1980, infant botulism has been the most common form of botulism reported in the United States. It is epidemiologically distinct from foodborne botulism, representing not ingestion of toxin preformed in contaminated foods but colonization (infection) of the intestine by spores of (21) C. Although infant botulism was first (22,23) described in 1976, earlier cases have been identified retrospectively, and its detection only in recent years might reflect advances in diagnostic capabilities rather than the emergence of a new clinical syndrome. The disease is characterized by the onset of constipation, which is followed shortly by neuromuscular paralysis that begins with the cranial nerves and progresses to peripheral and respiratory musculature. The spectrum in severity has ranged from mild lethargy and slowed feeding to severe hypotonia and respiratory insufficiency. Epidemiology Since the recognition of infant botulism in 1976, cases have been identified with increasing frequency. Since reporting began to stabilize in 1980, the average annual incidence of reported infant botulism in the United States has been approximately 1. Between 1976 and 1994, the incidence of infant botulism was highest in Delaware, Hawaii, Utah, and California (9. Infants hospitalized with the disease tend to have had higher birth weights than other infants, and their mothers tend to be white, older, and better educated than mothers in the general population. No congenital abnormalities have been shown to be associated with infant 9 botulism, and the children were generally healthy until the onset of botulism. Clustering of cases of infant botulism has been noted in some suburban areas in the eastern United States and in some small towns and rural areas in the (26-29) West. In a prototypical case, type B organisms, but no toxin, were isolated from honey fed to an infant with infant botulism whose fecal specimens contained type B organisms and toxin. In several studies, more than 20% of affected infants had (25,29,30) ingested honey before the onset of botulism. However, since most infants with infant botulism have had no exposure to honey, the risk (21,31) factors and vehicles of transmission of C. Investigators have also frequently noted environmental conditions that might expose infants directly to environmental sources of C. Infants hospitalized with botulism have also (25,31,33,34) more typically been breast-fed than have control infants. Breast-feeding is known to affect the fecal flora differently than formula feeding; in mice, the fecal flora have been shown to (35) be an important susceptibility factor in challenge experiments with C. Prevention and control the risk factors for infant botulism are poorly described, but possible sources of spores include foods and dust. Honey should not be fed to infants because it has been identified as a food (19) source. Neurologic findings are indistinguishable from those seen in foodborne botulism; however, gastrointestinal symptoms do not occur. Between 1943, when the syndrome was first recognized, through 1985, 33 cases of wound botulism were reported in the United States. Of these, 25 were laboratory confirmed; 17 cases were type A, 7 type B, and 1 a mixture of type A (37) and type B organisms. The wounds were usually deep and contained avascular areas; many patients had compound fractures, and four had extensive crush injuries of the hand. The median incubation 10 (38) period in cases of trauma was 7 days (range 4-21 days). Since 1980, wound botulism cases have occurred in persons who used illicit drugs; these were associated either with needle puncture (39) sites or with nasal or sinus lesions due to chronic cocaine sniffing. From 1986 through 1996, 78 cases of wound botulism were reported in the United States; the majority were linked to injectable drug use, particularly with so-called black tar heroin. Although there has been speculation since the 1920s, careful investigation has now demonstrated that some of these cases are caused by colonization of the gastrointestinal tract by C. Support for the diagnosis of botulism from intestinal colonization is provided by the demonstration of the prolonged excretion of toxin and C. In some cases of botulism strongly suspected of representing intestinal colonization, the patients had a history of gastrointestinal surgery or illnesses such as inflammatory bowel disease, which might (42) have predisposed them to enteric colonization. Clinical syndrome the clinical syndrome of botulism, whether foodborne, infant, wound, or intestinal colonization, is dominated by the neurologic symptoms and signs resulting from a toxin-induced blockade of the voluntary motor and autonomic cholinergic junctions and is quite similar for each (21,34,38,43) cause and toxin type. Incubation periods for foodborne botulism are reported to be as (44) short as 6 hours or as long as 10 days, but generally the time between toxin ingestion and onset (45) of symptoms ranges from 18 to 36 hours. The ingestion of other bacteria or their toxins in the improperly preserved food or changes in bowel motility are likely to account for the abdominal pain, nausea and vomiting, and diarrhea that often precede or accompany the neurologic symptoms of foodborne botulism. Dryness of the mouth, inability to focus to a near point (prompting the patient to complain of "blurred vision"), and diplopia are usually the earliest neurologic complaints. If the disease is mild, no other symptoms may develop and the initial symptoms will gradually resolve. In more severe cases, however, these initial symptoms may be followed by dysphonia, dysarthria, dysphagia, and peripheral-muscle weakness. If illness is severe, respiratory muscles are involved, leading to ventilatory failure and death unless supportive care is provided. A 2to 8-week duration of ventilatory support is common, although patients have required ventilatory support for up to 7 11 (43) months before the return of muscular function. Death occurs in 5%-10% of cases of foodborne botulism; early deaths result from a failure to recognize the severity of disease or from secondary pulmonary or systemic infections, whereas deaths after 2 weeks are usually from the (43) complications of long-term mechanical ventilatory management. Perhaps because infants are not able to complain about the early effects of botulinum intoxication, the neurologic dysfunction associated with infant botulism often seems to develop suddenly. The major manifestations are poor feeding, diminished suckling and crying ability, neck and peripheral weakness (the infants are often admitted as "floppy babies"), and ventilatory (21,24,34) failure. Constipation is also often seen in infants with botulism, and in some, has preceded the onset of neurologic abnormalities by many days. Loss of facial expression, extraocular muscle paralysis, dilated pupils, and depression of deep tendon reflexes have been reported more (34) frequently with type B than with type A infant botulism. Treatment with aminoglycoside (46) antimicrobial agents may promote neuromuscular weakness in infant botulism and has been (21,34) associated with an increased likelihood of the requirement of mechanical ventilation. The diagnosis is not difficult when it is strongly suspected, as in the setting of a large outbreak, but since cases of botulism most often occur singularly, the diagnosis may pose a more perplexing problem. Findings from many outbreaks have suggested that early cases are commonly misdiagnosed.

