Pariet
Order genuine pariet line
The but the large-scale anthropogenic production of phytoestrogens mechanisms are not understood gastritis symptoms vs ulcer symptoms order pariet without a prescription, but it has been suggested that in runoff from agricultural areas, wood pulp mill discharge, and phytoestrogens act as estrogen agonists and may increase spine sewage treatment plant effuent may still pose a threat to aquatic density and synapse formation in the hippocampus of adults. It Phytoestrogen content of foods consumed in Canada, including was also found in the investigation studies that rates of vaginal isofavones, lignans, and coumestan. Accessed June 9, 2016 Thus from the foregoing it is quite evident that diets rich in plant derived products may supply a variety of phytoestrogens capable 6. Phytoestrogen biological actions on mamma of producing a range of pharmacological effects in the human lian reproductive system and cancer growth. Endocrine Disruptors: Effects on Male and disease, increased risk of breast cancer and other symptoms Female Reproductive Systems. The pros and cons of phytoes health and their potential to be used as pharma foods, but the trogens. Role of Phytoestrogens as Nutraceuticals differences of phytoestrogens, their biological activities are in Human Health. In: Phytochemicals of nutraceutical impor also highly variable and there may be other effects that have tance. Dose-dependent effects of soy phytoestro gen genistein on adipocytes: Mechanisms of action. Development of antibodies against secoisolanciresinol Application to the immunolocalization of 28. Structure-radical 2016 scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Soy phytoestrogen effects on progesterone receptor and ovalbumin synthesis in the chick oviduct. Lignans and neolignans as European Poultry Conference, Verona, Italy, 10-14 September, lead compounds. Clinical studies show no effects of soy protein or isofavones on reproductive hormones in men: Results of a meta 21. The mutagenic and anti-mutagenic effects of the traditional phytoestrogen-rich herbs. Soybean antimicrobial peptides beta-defensin-2 synthesis in endometrial isofavones reduce postmenopausal bone resorption in female epithelial cells with lipopolysaccharides and polyinosinic Japanese immigrants in Brazil: A ten-week study. Breast cancer cell apoptosis Effect of long-term intervention of soy isofavones on bone with phytoestrogens is dependent on an estrogen deprived state. Genistein-selenium combination induces growth arrest in intakes of total and specifc lignans are associated with clinical prostate cancer cells. Prospective study on usual dietary phytoestrogens intake and cardiovascular disease 72. Toyohira Y, Ueno S, Tsutsui M, Itoh H, Sakai N, Saito cardiovascular disease and cancer. Preventive effects of phytoestrogens against postmenopausal osteoporosis as compared to the available therapeutic choices: An overview. The Effect of Soy Phytoestrogens on Bone Turn over Markers in Women during Early Menopausal Period. As there is no control during the pre-harvesting or post-harvesting stages of production other grains, crambe may be contaminated by fungi. Fungal overgrowth may lead to mycotoxins production and nutritional properties decrease of the grains. The aim of this study was to analyze the occur rence of fungi and mycotoxins according to pre-harvesting management. Fungal concentration was higher than that recommended by international regulations (3. More studies are required about quality of crambe grains, because may be strongly affected by the exposition to variable environmental conditions. But, considering low mycotoxin incidence and levels founded, the crambe proves to be a safe food to be exploited for animal nutrition. Crambe was introduced restricted use, distribution, and in Brazil for the production of biodiesel and to be used as forage. The inclusion of crambe reproduction in any medium, pro seeds was expected to correct the dry matter content and also increase the crude protein content vided the original work is properly of the silage (containing 37% of oil, 21% proteins, 13% fbers, magnesium, potassium and cited. The samples from the impacts from the disposal of this material on the environment. As happens with other cultures, crambe may be con taminated by fungi, which may jeopardize physical and nutri Determination of Water Activity tional features of the plant and produce mycotoxins, dangerous for human and animal health. Some strains were selected for species the relation between the plant and its endophytic mycobiota and identifcation. The main interaction that may8 macroscopic and microscopic features of samples and according lead to a contamination of crambe can be those with insects, with to taxonomic tables. Ten grains of crambe were disinfected and directly dis during the fnal stage of grains ripening or grains genotype. Mycotoxins of Fusarium species have been found to cause ma Plates were incubated at 25 C for seven days. All plates were jor damage, especially in cereals, and could frequently be asso assessed and results were expressed as percentage of infected ciated with pre-harvest cereal contamination. Fungal samples isolated by this technique were classifed climatic and meteorological assessment during cultivation to as according to the specifc taxonomic keys, described above. Mobile phase A: wa ter, mobile phase B: acetonitrile/water (95:5, v/v), the fow rate Sampling was 50 L/min. The sample was reconstituted in 400 L of the starting mobile phase, fltered and 20 L were injected. From genus the aW, temperatures and precipitation submitted to grains in the Penicillium were isolated the species: P. Areas with and without sunfower residues Fungal genus Number of samples Frequency (%) Aspergillus spp. Table 4: Mycotoxin levels in crambe seed samples from plants grown in areas with different levels of potassium fertilization taken from 5 seasons of crops (0, 15, 30, 60 and 90) and area with (C/R) and without (S/R) sunfower residues. All samples Calcination does not affect the population of Aspergil showed higher concentration of fungi than prescribed by inter lus spp. Also describe, periods of lower hu harvest showed higher fungal contamination, probably because midity of the ground are associated to higher frequency of As of the heavier rainfall during the three days before the harvest pergillus favus isolation. The samples of crambe grains from areas with sun fower residual relevance of the mycotoxins founded are corresponding with culture and from samples obtained from the second harvest (C2). The quality of crambe of feed, causing economic losses and damages to animal health. Mycotoxin research in to the exposure to adverse meteorological conditions may cause Brazil. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in 1. Accessed April 15, 2016 gem, da colheita e da secagem na qualidade sanitaria de amen doim da seca [In Portuguese]. Rumen degradation and intestinal digestion of cram cies: An Illustrated Manual for Identifcation. Utrecht, the Netherlands, England: Central Bureau Voor Schimmelcultures, Institute of the Royal Netherlands Academy 5. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Effects of temperature, water activity and incubation time on fungal growth and afatoxin B production by toxinogenic aspergillus1 favus isolates on sorghum seeds. Production of mycotoxins by Fusarium graminearum isolated from small cereals (wheat, triticale and barley) affected with scab disease in Southern Brazil. Risk Assessment and Safety Evaluation of Mycotoxins in Fruts: Mycotoxins in Fruits and Vegetables. Immunoaffnity column coupled with solution fuorometry or liquid chromatog raphy postcolumn derivatization for determination of afatoxins in corn, peanuts, and peanut butter: Collaborative study. Fungal and my cotoxins contamination in corn silage: Monitoring risk be fore and after fermentation.
Purchase cheap pariet on line
However gastritis diet ех buy pariet 20 mg without prescription, each step in the samPle history still must be asked, even if such forms are available for reference. Primary and backup communication procedures should be established prior to a remote trip. Allergies and Anaphylaxis when the body recognizes an allergen, a foreign substance that causes an allergic reaction, its immune system releases histamines and other chemicals for protection. However, a severe allergic reaction, called anaphylaxis, is life threatening and must be treated immediately or the person will die. Anaphylaxis it is important to know the signs and symptoms and be able to differentiate between a mild allergic reaction and a severe reaction (anaphylaxis). Altitude Illnesses altitude illnesses occur when people at a high altitude do not have enough oxygen in their blood because the air pressure is too low. Acute Mountain Sickness ams commonly occurs in a person who has recently reached an altitude of around 6500 to 8000 feet. High Altitude Pulmonary Edema HaPe is caused by fuid collecting in the air spaces of the lungs. Bone and Joint Injuries there are four main types of bone and joint injuries: n strains: overstretched muscles and/or tendons that attach muscles to bones n sprains: injuries to ligaments that hold bones together at joints n fractures: a break, chip or crack in a bone n dislocations: movement of a bone away from its normal position at a joint Because these injuries can look alike, you may have diffculty determining exactly which type of injury has occurred. Place a layer of gauze or cloth between the cold or ice pack and the skin to prevent damaging the skin. Splinting Follow these Principles: n in a wilderness or remote setting, chances are the person will need to be moved. Application of a Sling-and-Swathe (Sling and Binder) n support the injured body part above and below the site of the injury. Figure 6 32 wilderness and remote first aid emergency reference guide Application of an Anatomic Splint n support the injured body part above and below the site of the injury. Application of a Soft Splint n support the injured body part above and below the site of the injury. For a jaw fracture: n Hold the jaw in place with a wide wrap that goes around the head. For a lower arm fracture (including the wrist and hand): n secure the injured part to a well-padded, rigid support and place it in a sling-and-swathe. For a rib fracture: n Protect the injured rib by supporting the arm on the injured side with a sling-and-swathe. For a pelvis or hip fracture: n secure the person on a rigid litter (stretcher) before attempting a carry-out. For a leg fracture (including ankle and foot): n secure the injured part on a well-padded, rigid support that includes immobilization of the ankle and foot. Dislocations Follow these Principles: n the only treatment available for certain dislocations that occur in the wilderness is splinting in the most comfortable position. Figure 11 or n Have the injured person perform a similar technique on him or herself right away: standing or sitting, the person should pull the injured arm straight and forward, away from the body, by gripping the wrist with the opposite hand (figure 12). Figure 12 n Place the injured arm in a sling-and swathe as soon as the shoulder is returned to its normal position. For a kneecap dislocation: n apply gentle traction to the leg to straighten it out. Chest Injuries any signifcant injury to the chest may lead to diffculty breathing, a potentially serious and life-threatening problem. Figure 5 use a modifed jaw thrust to open or maintain airway if trained and if needed. Special Considerations: Focused Spine Assessment if the moi caused you to suspect a spinal injury but a full assessment did not reveal any signs and symptoms, perform a focused spine assessment. Figure 1 n if the person cannot exercise muscles easily, try to keep him or her warm by: insulating him or her from the ground. Figure 2B injuries and illnesses 61 the hypothermia wrap should resemble a cocoon in that it only opens to the mouth and nose. Figure 1 66 wilderness and remote first aid emergency reference guide n if the person is unconscious and not breathing, immediately begin cardiopulmonary resuscitation (cPr) or use an automated external defbrillator (aed), if one is available. Submersion Incidents (Drowning) the frst step when dealing with any emergency is to determine that the scene is safe for the rescuer. Reach out to the person with a hand, foot, clothing, stick, paddle or anything that allows you to remain safely on land or in a boat (figure 4). Keep the object between you and the victim to help prevent the victim from clutching at you in a panic. Figure 6 70 wilderness and remote first aid emergency reference guide when the victim has grasped the object or the line, slowly pull him or her to safety. Row to the person, or get to the person in some sort of watercraft, using reaching or throwing devices as appropriate, with safety as a top priority (figure 7). Figure 3 Tourniquets use a tourniquet on an arm or leg only if blood loss is uncontrolled by direct pressure or if direct pressure is not possible. Figure 4 74 wilderness and remote first aid emergency reference guide n tie a short, sturdy stick or another rigid object into the material and twist it (called the windlass technique) (figure 5). Wound Cleaning after bleeding is controlled, the wound should be properly cleaned, closed and dressed to prevent infection, promote healing and reduce scarring. Wound Dressing and Closing Lacerations lacerations are cuts through the skin that have even or ragged edges (figure 7). Figure 8 if using closure strips, apply one end of one strip to one side of the wound and another to the opposite side. Figure 11 Chafng chafng occurs from excess friction, often in the groin area and between the thighs. Figure 12 n if bleeding persists, have the person continue pinching the nose shut for another 10 minutes and repeat until bleeding stops. For a knocked-out tooth: n Bleeding can be controlled by biting down on a rolled gauze pad. With chronic infection: n intermittent episodes of: mushy, foul-smelling stools. Lower right Lower left quadrant quadrant Figure 2 special situations 87 Asthma Attack asthma is a condition that narrows the air passages in the lungs and makes breathing diffcult. Keep the frostbitten part in the water until normal color returns and it feels warm. Immersion Foot immersion foot occurs when feet have been exposed to more than 12 hours of cold, wet conditions. In the wilderness or remote areas, examples of confned spaces include: n crevasses. Diabetic Emergency people with diabetes sometimes become ill because there is too much or too little sugar in their blood. Emergency and Non-Emergency Moves one of the most dangerous threats to a seriously injured person is unnecessary movement. Pack-Strap Carry to move either a conscious or an unconscious person when you do not suspect a head, neck or back injury: 1. Figure 3 Clothes Drag to move a person who may have a head, neck or back injury: 1. Figure 4 Blanket Drag to move a person in an emergency situation when equipment is limited: 1. Figure 6 Stretchers a stretcher (litter) can be used to move a person a short distance to a better site for giving care. For a jellyfsh sting: n irrigate the injured part with vinegar for at least 30 seconds. Seizures when the normal functions of the brain are disrupted by injury, disease, fever, poisoning or infection, the electrical activity of the brain becomes irregular. Amputation amputation is the complete removal or severing of an external body part.
Order pariet online
The chairperson should gastritis upper gi order pariet 20mg line, however, be a physician knowledgeable in transfusion medicine. The committee should establish guidelines for administration of each of the blood components transfused in the institution, using current medical literature as a resource. The transfusion guidelines should be approved by the Medical Staf prior to implementation. Transfusion guidelines are intended to remind ordering physicians of the transfusion practices for which there is general support and clinical trial evidence. Guidelines cannot be expected to cover every instance in which a transfusion is indicated. In every case, however, the rationale for transfusion should be clearly documented in the medical record. Process: the review of transfusions can be done prospectively by transfusion service personnel (before blood is issued) or retrospectively by the Transfusion Committee (after blood is issued) for certain high cost blood products, prospective review may be appropriate to prevent unnecessary transfusions. Similarly, prospective review of potentially inappropriate orders, for example, an order for platelet transfusion to a patient with thrombotic thrombocytopenic purpura or an order for four units of red blood cells for a child, may also require review prior to blood issue. For most transfusions and blood products, 45 however, involving large numbers of transfusions and patients, retrospective reviews are adequate and most commonly used. Applicable lab or clinical results before and after transfusion Trained hospital quality assurance or compliance staf can do chart or electronic record reviews, using the approved transfusion guidelines developed by the committee. When there are questions about the indications and results of a transfusion, the clinical records should be peer reviewed or reviewed at the transfusion committee meeting. If the letter is ignored or if repeated unjustifed transfusion practices are noted, a department chair or credentialing committee may need to be involved in the review process. Monitors: Blood usage should be monitored by whichever parameters are most useful for the institution: by physician, by clinical department, by diagnosis (Diagnosis-Related Groups), or by surgical procedures In addition, the Transfusion Committee must ensure that blood is administered correctly. The wastage of all blood components, both allogeneic and autologous, should be monitored. The committee must also ensure that a mechanism exists for reporting and evaluation of suspected transfusion transmitted diseases. Reports: the Transfusion Committee or its equivalent, should document activities by minutes and generate reports of its work for submission to other entities of the hospital. The intent of this reporting is to provide other peer review committees with the results of reviews of transfusion related patient care. These minutes can be protected from inappropriate legal discovery as a critical component of an institutions quality monitoring program. Summary: Hospitals are required to review blood transfusion practices and adverse outcomes. Accrediting and regulatory agencies do not specify how this peer review function is accomplished, as long as it is being performed. It is simply a matter of having appropriate policies and procedures in place, reviewing and revising them as necessary, and monitoring that they are followed. General the following side efects and hazards pertain to transfusion of Whole Blood or any component prepared from blood collected from individual donors. Hemolytic transfusion reaction, the destruction of transfused red cells, is discussed in detail in the section on red-cell-containing components. Febrile nonhemolytic reaction is typically manifested by a temperature elevation of 1 C or 2 F occurring during or shortly after a transfusion and in the absence of any other pyrexic stimulus. This may refect the action of antibodies against white cells or the action of cytokines, either present in the transfused component or generated by the recipient in response to transfused elements. Febrile reactions may accompany about 1% of transfusions; and they occur more frequently in patients previously alloimmunized by transfusion or pregnancy. Patients who experience repeated, severe febrile reactions may beneft from receiving leukocyte reduced components. If these reactions are due to cytokines in the component, prestorage leukocyte reduction may be benefcial. Allergic reactions usually occur as urticaria, but may also include wheezing or angioedematous reactions. No laboratory procedures are available to predict or prevent these reactions, which usually respond to antihistamines or, in severe cases, corticosteroids or epinephrine. Anaphylactoid reactions, characterized by autonomic dysregulation, severe dyspnea, pulmonary and/or laryngeal edema, and bronchospasm and/or laryngospasm, are a rare but dangerous complication requiring immediate treatment with corticosteroids and epinephrine. The majority of these reactions have been reported in IgA-defcient patients who have IgA antibodies of the IgE class. Such patients may not have been previously transfused and may develop symptoms after infusion of very small amounts of IgA containing plasma, in any blood component. Delayed hemolytic reaction is described in detail in the section on red-cell-containing components. Primary immunization does not become apparent until days or weeks after the immunizing event, and does not usually cause symptoms or physiologic changes. If components that express the relevant antigen are subsequently transfused, there may be accelerated removal of cellular elements from the circulation and/or systemic symptoms. Clinically signifcant antibodies to red cell antigens will ordinarily be detected by pretransfusion testing. Alloimmunization to antigens of white cells, platelets, or plasma proteins can only be detected by specialized testing. While the immune specifcity may be to a platelet specifc antigen the patient lacks, autologous and allogeneic platelets are destroyed. Transmission of infectious disease may occur because this product is made from human blood. For other infectious agents, there are no 50 routinely available tests to predict or prevent disease transmission. All potential blood donors are subjected to stringent screening procedures intended to reduce to a minimum the risk that they will transmit infectious agents. Bacterial contamination occurs rarely but can cause acute, severe, sometimes life-threatening efects. Platelet components stored at room temperature, previously frozen components thawed by immersion in a waterbath, and red cell components stored for several weeks at 1-6 C have been implicated. Both gram-positive and gram-negative organisms have been identifed as causing septic reactions. Organisms capable of multiplying at low temperatures and those using citrate as a nutrient are most often associated with red cell contamination. A variety of pathogens, as well as skin contaminants, have been found in platelet concentrates. Endotoxemia in recipients has resulted from multiplication of Yersinia enterocolitica in stored red-cell-containing components. Prompt recognition of a possible septic reaction is essential, with immediate discontinuation of the transfusion and aggressive therapy with broad-spectrum antimicrobials and vasopressor agents, if necessary. Circulatory overload, leading to pulmonary edema, can occur after transfusion of excessive volumes or at excessively rapid rates. This is a particular risk in the elderly and in patients with chronic severe anemia in whom low red cell mass is associated with high plasma volume. Small transfusion volumes can precipitate symptoms in at-risk patients who already have a positive fuid balance. Pulmonary edema should be promptly and aggressively treated, and infusion of colloid preparations, including plasma components and the suspending plasma in cellular components, reduced to a minimum. Rapid infusion of large volumes of cold blood can depress body temperature, and the danger is compounded in patients experiencing shock or surgical or anesthetic manipulations that disrupt temperature regulation. Metabolic complications may accompany large volume transfusions, especially in patients with liver or kidney disease. Patients with severe liver disease or those with circulatory collapse that prevents adequate hepatic blood fow, may have physiologically signifcant hypocalcemia after rapid, large-volume transfusion. Citrated blood administered rapidly through central intravenous access may reach the heart so rapidly that ventricular arrhythmias occur.
Order pariet with mastercard
Continue to provide nal trexone for patients who were already receiving Naltrexone Offce-based treatment gastritis diet quizzes order cheapest pariet and pariet, it from some other setting. If no the services and requirements typical of this immediate openings are available, consider treatment setting. These programs range is indicated, determine whether the residential from low intensity (individual or group counseling program allows patients to continue their opioid once to a few times a week) to high intensity receptor agonist medication while in treatment. Providing a plan maximizes the likelihood of continuity of naloxone prescription and overdose prevention care after discharge. Follow up with the patient later to determine Make referrals to mutual-help groups. Doing Patients may wish to participate in mutual so increases the chances of a successful referral. Drug Addiction Treatment Act of 2000 Patients with depression, anxiety disorders, and legislation requires that buprenorphine pre other mental disorders may be more likely to scribers be able to refer patients to counseling, 81 succeed in addiction treatment if those condi but making referrals is not mandatory. However, four refer the patient to mental health services as randomized trials found no extra beneft to appropriate. Opioids (including prevention, identifcation, and response (Exhibit prescription opioids and heroin) killed 2. For information about all forms of naloxone, all opioid overdose deaths was 46,800. At the time of this publication, only Disorders: Provides comprehensive the draft (not the fnal) recommendation treatment guidance for individuals with statement is available. Continued opioid use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of opioids. Results from the 2018 National interventions to reduce unhealthy alcohol use in Survey on Drug Use and Health: Detailed tables. Recommendation Statement: Unhealthy Alcohol Use 3 American Society of Addiction Medicine. Diagnostic Page/Document/RecommendationStatementFinal/ and statistical manual of mental disorders (5th ed. The Alcohol Use Disorders 6 Substance Abuse and Mental Health Services Identifcation Test: Guidelines for use in primary Administration. Medication for the treatment Performance of the Tobacco, Alcohol, Prescription of alcohol use disorder: A brief guide. Primary care validation of a single Alcohol and public health: Alcohol-Related Disease question alcohol screening test. Ultra-rapid screening for substance and the Heaviness of Smoking Index in two adult use disorders: the Alcohol, Smoking and Substance population samples. A single-question screening test for Nonmedical Use of Prescription Drugs: Screening. Screen of drug use: screening Diagnostic accuracy of a new brief tool for primary care. Retrieved November 21, 2017, from Evidence from the National Survey of Drug Use and Diagnostic behavioral interventions in buprenorphine maintenance and statistical manual of mental disorders (5th ed. Retrieved October 16, 2017, from American Journal of Medicine, 126(1), 74. Counseling plus buprenorphine-naloxone and statistical manual of mental disorders (5th ed. Comparison of behavioral treatment conditions or methadone maintenance for opioid dependence. Current status of co-occurring mood and substance use Cochrane Database of Systematic Reviews, 4, disorders: A new therapeutic target. Randomized trial of long-acting sustained Medication-assisted treatment models of care for release naltrexone implant vs oral naltrexone or opioid use disorder in primary care settings. Alcohol Use Disorders 93 Substance Abuse and Mental Health Services Identifcation Test. Unlike with full agonists, increasing their dose in an opioid-tolerant individual may not produce additional effects once they have reached their maximal effect. Pharmacology Opioid receptor Opioid receptor partial Opioid receptor Opioid receptor partial agonist agonist agonist antagonist Reduces opioid Reduces opioid Reduces opioid Blocks euphoric withdrawal and withdrawal and craving; withdrawal and effects of self craving; blunts or blunts or blocks craving; blunts or administered illicit blocks euphoric euphoric effects of blocks euphoric opioids through effects of self self-administered illicit effects of self opioid receptor administered opioids through cross administered occupancy. Subdermal dispersible tablet release formulation implants every 6 or powder (unless in abdominal region months, for up to patients can take for patients treated 1 year, for stable some home). Any are available via restricted pharmacy can distribution programs and fll a prescription are not available in retail for sublingual pharmacies. Short-term counselors should be familiar with pilot studies show that offering naltrexone under methadone. Their patients may be these circumstances can increase treatment engagement after release. They do not apply to with central nervous system depressants such as pain treatment using buprenorphine benzodiazepines or alcohol. Patients should be urine drug testing data from 1,027 participants informed of the risks and benefts of discontin and 4 studies with self-reported drug use from uing medication. Discontinuing patients randomly assigned to start buprenor medication increases risk of return to substance phine had signifcantly lower return-to-use use and overdose death.
Diseases
- Deal Barratt Dillon syndrome
- Nicolaides Baraitser syndrome
- Brachyd
- 3 beta hydroxysteroid dehydrogenase deficiency
- Hyperimmunoglobulin E - reccurrent infection syndrome
- Bardet Biedl syndrome, type 3
- Richter syndrome
- Brachioskeletogenital syndrome
- Anophthalmia Waardenburg syndrome
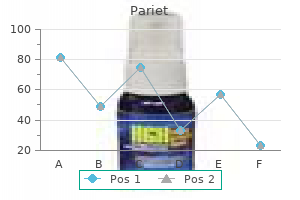
Purchase 20 mg pariet with mastercard
We are not currently planning to hold an advisory committee meeting to discuss this application gastritis treatment and diet generic 20mg pariet with visa. We request that you submit the following information by close of business, December 28, 2016: 1. Confirm that all the batches of the product used in the pivotal clinical studies, and the to-be marketed product will be manufactured at the same site. Provide the appropriate formulation bridging data if the clinical batches were manufactured at multiple sites. During our preliminary review of your submitted labeling, we have identified the following labeling issues and have the following labeling comments or questions: 1. We request that you resubmit labeling (in Microsoft Word format) that addresses these issues by January 6, 2017. While we anticipate that any response submitted in a timely manner will be reviewed during this review cycle, such review decisions will be made on a case-by-case basis at the time of receipt of the submission. Provide K-M analysis with exposure quartile and multivariate cox proportional hazard analysis to adjust for the other prognostic factors. Please further evaluate whether the required dose reduction is an optimal approach to manage the anemia given the potential loss of efficacy at lower dose and the existing other measures. Thank you, Jeannette Dinin Regulatory Project Manager Division of Oncology Products 1 Office of Hematology and Oncology Products Center for Drug Evaluation and Research U. We ask for an analysis without the adjustment of baseline demographic values and stratification factors. In addition, we may be coming back with an additional question in the next day or so. I have bolded the query number and the question we have just to make it easier to find. The original longitudinal model in the report included stratification factors, baseline value, treatment, visit, treatment-by-visit interaction and individual patient effect. We would like to clarify what is meant by the baseline demographic values since the model did not include any demographic variables. Does the agency mean removing the baseline score for the Health Outcome instrument score This is to check whether the investigator followed the protocol specified progression criteria or not. For the longitudinal analysis of each instrument, please perform an analysis without adjustment by baseline demographic values and stratification factors. We acknowledge receipt of your correspondence, dated and received November 2, 2016, requesting a review of your proposed proprietary name, Zejula. If you have any questions regarding the contents of this letter or any other aspects of the proprietary name review process, please contact me at (301) 796-0942. For any other information regarding this application, contact Jeannette Dinin, Regulatory Project Manager, in the Office of New Drugs at (240) 402-4978. Please perform the following analyses using data prior to or on the clinical cutoff date, May, 30 2016. This is to check whether the central reviewers followed the protocol specified progression criteria or not. The meeting will occur on: Meeting Date and Time: November 28, 2016; 10:30-11:30 am Meeting Location: White Oak, Building 22, Room 2205 Because space is limited please limit your attendees to approximately 12 participants. Preferably, the meeting would take place as soon as possible once the application has been submitted so that the review team can become familiar with your application. These are general comments and we acknowledge that individual applications have unique characteristics. If you believe some comments are inapplicable to your application and therefore your presentation and/or you believe that other information is relevant, adjust your presentation accordingly. Application Orientation Presentation meetings are generally one hour in length, including time for discussion and Q & A (approximately 35-40 minutes of presentation and 25-20 minutes for discussion). The primary focus of the presentation should be on clinical (with clinical sections presented first) with highlights of other sections to follow. Drug/biologic characteristics, including what makes the drug/biologic unique, mechanism of action. Listing of registration trial(s), to support marketing/licensing application, as well as Phase 1 and Phase 2 trials to support application. If accelerated approval, design of the confirmatory trial(s) that will be ongoing at the time of accelerated approval and a timetable of when confirmatory trial(s) will be completed and final clinical study report(s) submitted. Regulatory history, including the following: Orphan Drug designation, Fast Track designation Foreign Regulatory history: Where/when approved and for what indications, whether there are pending applications with foreign regulators, Risk management plans in foreign countries. You should also present results of the following, as appropriate: Clinical study sites (foreign or domestic) Biomarker development for population selection (if applicable) Assay validation (if applicable) 120-day Safety update: Plans for 120-day Safety update, including how many additional patients will be included in safety update and from which studies. Statistics: Study design, description of planned analyses, efficacy analyses, safety analyses, subpopulation analyses of safety and efficacy (age, sex, race, concurrent therapy, number of prior treatments, region/country), length of follow-up, handling of missing data 10. Please note that secure email may not be used for formal regulatory submissions to applications. Confirm whether this is the case (or not), and provide us with the address, (b) (4) contact person, and phone number for. We will need the charter that was used by the blinded central radiology review committee. Safety Clinical Cardiac Safety Describe total number of clinical trials and number of subjects at different drug exposure levels. We also refer to your August 25, 2016, request for Breakthrough Therapy designation. Please note that if the clinical development program does not continue to meet the criteria for Breakthrough Therapy designation, we may rescind the designation. At this point in your drug development program, holding this initial Breakthrough Therapy meeting is not necessary. However, please contact the Regulatory Project Manager noted below to determine if any information is required at this time to expedite the review of your breakthrough designated product. However, if you have Fast Track designation you will be able to submit portions of your application under the Fast Track program. If you have any questions, contact Jeannette Dinin, Regulatory Project Manager, at (240) 402-4978 or email Jeannette. Endpoint does not assess or is not plausibly related to a serious aspect of the disease. No or minimal clinically meaningful improvement as compared 2 to available therapy / historical experience. Ovarian cancer is the fifth overall cause for cancer death in women, representing 5% of all cancer deaths in women. It is also the deadliest of gynecologic cancers: an estimated 14,240 deaths are expected in 2016 in the United States. Early stage ovarian cancer is often asymptomatic; therefore, it is often first detected in advanced stages when prognosis is poor. The 5-year overall survival rate of ovarian cancer patients is 46% across all stages, but only 29% in patients diagnosed with distant metastatic disease. Although platinum-based chemotherapy is effective at inducing an initial response, ovarian cancer will recur in the majority of women. After relapse, patients respond moderately or poorly to subsequent chemotherapy, with later lines of therapy leading to progressively shorter platinum-free intervals. There previously have been no approvals in the maintenance setting for advanced ovarian cancer. Describe the endpoint(s) that are accepted by the Division as clinically significant (outcome measures) for patients with the disease. Describe any other biomarkers that the Division would consider likely to predict a clinical benefit for the proposed indication even if not yet a basis for accelerated approval.
Cheap pariet line
Health care professionals with cold sores who have contact with infants should cover and not touch their lesions and should comply with hand hygiene policies gastritis diet karbo purchase pariet overnight. Consideration of suppressive antiviral therapy should be limited to athletes with a history of recurrent herpes gladiatorum or herpes labialis to reduce the risk of reactivation dur ing wrestling season. Typical radiographic fndings include diffuse interstitial or reticulonodular pulmonary infltrates and hilar or mediastinal adenopathy. Chronic cavitary pulmonary histoplasmosis occurs most often in older adults and can mimic pulmonary tuberculosis. Mediastinal involvement, usually a complication of pulmonary histoplasmosis, includes mediastinal lymphadenitis, which can cause airway encroachment in young children. Infammatory syndromes (pericarditis and rheumatologic syndromes) also can develop; erythema nodosum can occur in adolescents and adults. Infection is acquired through inhalation of conidia from soil, often contaminated with bat guano or bird droppings. The inoculum size, strain virulence, and immune status of the host affect severity of illness. Prior infection confers partial immunity; reinfection can occur but requires a larger inoculum. Demonstration of typical intracellular yeast forms by examination with Gomori methenamine silver or other stains of tissue, blood, bone marrow, or bronchoalveolar lavage specimens strongly supports the diagnosis of histoplasmosis when clinical, epide miologic, and other laboratory studies are compatible. If the result initially is positive, the antigen test also is useful for monitoring treatment response and, after treatment, identifying relapse. Serologic testing also is available and is most useful in patients with subacute or chronic pulmonary disease. Cross-reacting antibodies can result from Blastomyces dermatitidis and Coccidioides species infections. The immunodiffusion test is more specifc than the complement fxa tion test, but the complement fxation test is more sensitive. Although safety and effcacy of itraconazole for use in children have not been established, anecdotal experience has found it to be well tolerated and effective. If the patient is symptomatic for more than 4 weeks, itraconazole should be given for 6 to 12 weeks, although the effectiveness of this treatment is not well documented. For severe acute pulmonary infections, treatment with amphotericin B is recommended for 1 to 2 weeks. Methylprednisolone during the frst 1 to 2 weeks of therapy can be used if respira tory complications develop. However, mediastinal adenitis that causes obstruction of a bronchus, the esophagus, or another mediastinal structure may improve with a brief course of corticosteroids. For treatment of progressive disseminated histoplasmosis in a nonimmunocompro mised infant or child, amphotericin B is the drug of choice and is given for 4 to 6 weeks. An alternative regimen uses induction with amphotericin B therapy for 2 to 4 weeks and, when there has been substantial clinical improvement and a decline in the serum concen tration of histoplasmosis antigen, oral itraconazole is administered for 12 weeks. Exposure to soil and dust from areas with signifcant accumulations of bird and bat droppings should be avoided, especially by immunocompromised people. If exposure is unavoidable, it should be minimized through use of appropriate respiratory protec tion (eg, N95 respirator), gloves, and disposable clothing. Old structures likely to have been contaminated with bird or bat droppings should be moistened thoroughly before demolition. Guidelines for preventing histoplasmosis have been designed for health and safety professionals, environmental consultants, and people supervising workers involved in activities in which contaminated materials are disturbed. Pneumonitis associated with migrating larvae is uncommon and usually mild, except in heavy infections. Blood loss secondary to hookworm infection develops 10 to 12 weeks after initial infection and symptoms related to serious iron-defciency anemia can develop in long-standing moder ate or heavy hookworm infections. Hookworms are prominent in rural, tropical, and subtropical areas where soil contamination with human feces is common. N americanus is predominant in the Western hemisphere, sub-Saharan Africa, Southeast Asia, and a number of Pacifc islands. A duodenale transmission can occur by oral ingestion and possibly through human milk. Approximately 5 to 8 weeks are required after infection for eggs to appear in feces. A direct stool smear with saline solution or potas sium iodide saturated with iodine is adequate for diagnosis of heavy hookworm infection; light infections require concentration techniques. Quantifcation techniques (eg, Kato Katz, Beaver direct smear, or Stoll egg-counting techniques) to determine the clinical signifcance of infection and the response to treatment may be available from state or reference laboratories. Although data suggest that these drugs are safe in children younger than 2 years of age, the risks and benefts of therapy should be con sidered before administration. Reexamination of stool specimens 2 weeks after therapy to deter mine whether worms have been eliminated is helpful for assessing response to therapy. Wearing shoes may not be fully protective, because cutaneous exposure to hookworm larvae over the entire body surface of children could result in infection. Three distinct genotypes have been described, although there are no data regarding antigenic variation or distinct serotypes. In temperate climates, seasonal clustering in the spring associated with increased transmission of other respiratory tract viruses has been reported. However, prolonged shedding of virus in respira tory tract secretions and in stool may occur after resolution of symptoms, particularly in immune-compromised hosts. Appropriate hand hygiene, particularly when handling respiratory tract secretions or diapers of ill children, is recommended. Roseola is distin guished by the erythematous maculopapular rash, which appears once fever resolves and can last hours to days. The clinical circumstances and manifestations of reactivation in healthy people are unclear. Virus-specifc maternal antibody, which is present uniformly in the sera of infants at birth, provides transient partial protection. However, seroconversion from negative to positive in paired sera is good evi dence of recent primary infection. Studies from areas with endemic infection have suggested transmission may occur by blood transfusion, but in the United States, such evidence is lacking. Three principal genes (gag, pol, and env) encode the major structural and enzymatic proteins, and 6 acces sory genes regulate gene expression and aid in assembly and release of infectious viri ons. Increased serum immunoglobulin (Ig) concentrations of all isotypes, particularly IgG and IgA, are manifes tations of the humoral immune dysfunction, but they are not directed necessarily at spe cifc pathogens of childhood. Specifc humoral responses to antigens to which the patient previously has not been exposed usually are abnormal; later in disease, recall antibody responses, including responses to vaccine-associated antigens, are slow and diminish in magnitude. A small proportion (less than 10%) of patients will develop panhypogamma globulinemia. Latent virus persists in peripheral blood mononuclear cells and in cells of the brain, bone mar row, and genital tract even when plasma viral load is undetectable. Only blood, semen, cervicovaginal secretions, and human milk have been implicated epidemiologically in transmission of infection. Transmission has been documented after contact of nonin tact skin with blood-containing body fuids. The introduction of complimentary foods should occur after 6 months of life, and breastfeeding should continue through 12 months of life. Sometimes, T-lymphocyte counts do not decrease until late in the course of infection. Data from both observational studies and clinical trials indi-2 cate that very early initiation of therapy reduces morbidity and mortality compared with starting treatment when clinically symptomatic or immune suppressed.
Purchase 20mg pariet with visa
Patient assessment should begin with attention to the primary survey gastritis endoscopy order pariet cheap, looking for evidence of circulatory collapse and ensuring effective respirations a. The patient suffering from moderate or severe hypothermia may have severe alterations in vital signs including weak and extremely slow pulses, profound hypotension and decreased respirations b. The rescuer may need to evaluate the hypothermic patient for longer than the normothermic patient (up to 60 seconds) 298 3. Mild: vital signs not depressed normal mental status, shivering is preserved; body maintains ability to control temperature b. Maintain patient and rescuer safety the patient has fallen victim to cold injury and rescuers have likely had to enter the same environment. Remove the patient from the environment and prevent further heat loss by removing wet clothes and drying skin, insulate from the ground, shelter the patient from wind and wet conditions, and insulate the patient with dry clothing or a hypothermia wrap/ blanket. Cover the patient with a vapor barrier and, if available, move the patient to a warm environment b. Hypothermic patients have decreased oxygen needs and may not require supplemental oxygen i. Provide beverages or foods containing glucose if feasible and patient is awake and able to manage airway independently d. Vigorous shivering can substantially increase heat production shivering should be fueled by caloric replacement. Monitor frequently if temperature or level of consciousness decreases, refer to Severe Hypothermia, below g. The recommended fluid for volume replacement in the hypothermic patient is normal saline h. If alterations in mental status, consider measuring blood glucose and treat as indicated (treat per Hypoglycemia or Hyperglycemia guidelines) and assess for other causes of alterations of mentation i. If esophageal temperature monitoring is not available or appropriate, use an epitympanic thermometer designed for field conditions with an isolating ear cap iii. Rectal temperatures may also be used, but only once the patient is in a warm environment rectal temperatures are not reliable or suitable for taking temperatures in the field and should only be done in a warm environment (such as a heated ambulance) b. Care must be taken not to hyperventilate the patient as hypocarbia may reduce the threshold for ventricular fibrillation in the cold patient ii. Indications and contraindications for advanced airway devices are similar in the hypothermic patient as in the normothermic patient c. Prevent further heat loss by removing the patient from the environment and removing wet clothes and drying skin, insulate from the ground, shelter the patient from wind and wet conditions, and insulate the patient with dry clothing or a hypothermia wrap/ blanket. Cover the patient with a vapor barrier and, if available, move the patient to a warm environment d. Chemical or electrical heat sources should never be applied directly to the skin ii. Attempt to keep the patient in the horizontal position, especially limiting motion of the extremities to avoid increasing return of cold blood to the heart ii. Once in a warm environment, clothing should be cut off (rather than removed by manipulating the extremities) 300 iii. Move the patient only when necessary such as to remove the patient from the elements f. If the patient has evidence of frostbite, and ambulation/travel is necessary for evacuation or safety, avoid rewarming of extremities until definitive treatment is possible. Additive injury occurs when the area of frostbite is rewarmed then inadvertently refrozen. If warm water is not available, rewarm frostbitten parts by contact with non-affected body surfaces. If blisters are causing significant pain, and the provider is so trained, these may be aspirated, however, should not be de-roofed. Given the additive effects of additional cold stress, the patient should be removed from the cold environment as soon as operationally feasible 2. In patients suffering from moderate to severe hypothermia, it is critical to not allow these patients to stand or exercise as this may cause circulatory collapse 3. In patients who are unresponsive, or unable to recognize a developing injury, please check the area in which the heating pad is placed regularly to ensure no tissue damage occurs. The following are contraindications for initiation of resuscitation in the hypothermic patient: a. The patient exhibits signs of being frozen (such as ice formation in the airway) c. Avalanche victims buried for 35 minutes or longer with airway obstruction by ice or snow 2. Fixed and dilated pupils, apparent rigor mortis, and dependent lividity may not be contraindication for resuscitation in the severely hypothermic patient 3. The mainstay of therapy in severe hypothermia and cardiac arrest should be effective chest compressions and attempts at rewarming Chest compressions should be provided at the same rate as in normothermic patients 4. The temperature at which defibrillation should first be attempted in the severely hypothermic cardiac arrest victim and the number of defibrillation attempts is unclear. There are different approaches regarding resuscitation of the hypothermic arrest patient. It is noted that the likelihood of successful defibrillation increases with every one-degree increase in temperature d. Manage the airway per standard care in cardiac arrest victims [see Cardiac Arrest guideline] a. In the absence of advanced airways, ventilate the patient at the same rate as a normothermic patient b. If the patient has an advanced airway, ventilate at half the rate recommended for a normothermic patient to prevent hyperventilation. Patients with severe hypothermia and arrest may benefit from resuscitation even after prolonged downtime, and survival with intact neurologic function has been observed even after prolonged resuscitation Patients should not be considered deceased until rewarming has been attempted 9. If a hypothermic patient clearly suffered cardiac arrest and subsequently became hypothermic afterward with prolonged down time between arrest and rescue, there is no rationale for initiating resuscitation and warming the patient Pertinent Assessment Findings 1. Measure of patients who received treatment to correct their hypoglycemia o Trauma-01: Pain assessment of injured patients. Recognizing that pain is undertreated in injured patients, it is important to assess whether a patient is experiencing pain 303 References 1. Wilderness Medical Society guidelines for the prevention and treatment of frostbite. Wilderness Medical Society practice guidelines for the prevention and treatment of frostbite: 2014 update. Pennsylvania Statewide Advanced Life Support Protocols: Hypothermia/cold injury/frostbite.
Cheap 20mg pariet mastercard
Bradykinesia and muscular rigidity Apraxic speech: prefrontal motor dysfunction gastritis diet контакт purchase discount pariet. Facial muscle weakness slurred speech Dysphonia: huskiness with decreased volume. Assess cough Presenting: Describe findings in terms of: Hearing/dysarthria Comprehension Is speech comprehensible Due to incoordination of tongue and face muscles Limbs: power normal, may be subtle tone. Tremour with testing coordination will be: Coarse: comes from incoordination of proximal muscles Intention: gets worse on approaching target (as opposed to postural/action tremour which is worse on sustained posture) Upper Limb: Pronator drift: Affected side arm drift upwards. If negative wrist and elbows Light touch Cape sensory loss (neck, shoulders, arms) syringomyelia, shield loss (front of chest) Sensory loss of dorsolateral forearm, thumb and index finger C7 Radiculopathy: pain from neck, shoulder, arm and forearm. Pain on sole of foot when walking, weakness of toe plantars S1 Radiculopathy: Pain in back, buttock, thigh, leg, and foot, numbness of the lateral border of the foot. Mild weakness of eversion and dorsiflexion, depressed ankle jerk L5 Radiculopathy: Pain in back, buttock, thigh, leg, and foot, numbness of medial border of the foot and big toe, weakness of inversion and dorsiflexion. No reflex change Common peroneal nerve lesion from compression at the fibula head: Painless, severe weakness of dorsiflexion and eversion, with normal inversion, and numbness on the lateral foot and dorsum of the foot. Policy Laboratory labels should uniquely identify the patient, capture the date and time at which a specimen was obtained, and identify the individual responsible for the collection of the specimen. Purpose the labeling of laboratory specimens is critical to ensuring the appropriate matching of specimen and subsequent test results to the respective patient. Specimens not labeled with Sunquest barcode labels should arrive in the laboratory with a requisition. If an unlabeled specimen is received in the laboratory, the following protocol will be observed: 1. As a witness, it is the responsibility of laboratory personnel to make sure the form is completely and correctly filled out. Mislabeled specimens that are received in the laboratory will be processed according to the Reference Lab Unlabeled Specimen Policy. Policy the laboratory test requisition policy ensures that the laboratory is carrying out the orders as directed by the physician and routes the results to the appropriate location. Purpose In the absence of a Sunquest barcode label, a requisition must accompany the specimen to the laboratory for testing. This document specifies which tests the laboratory is to perform along with the name of the ordering physician. Finally, for microbiology, histology and cytology specimens, the requisition serves to document the specimen type or body site, which ensures that appropriate processing occurs for that type of specimen. The person completing the requisition should ensure that the required information above is provided on the requisition. The requisition must accompany the sample to the laboratory for the testing process to begin. Policy Unlabeled Reference Lab specimens will be processed according to the following procedure. Any mislabeled specimens will be accessioned using the name indicated on the specimen and not the name on the requisition. Specimens found to be unlabeled should be accessioned in Sunquest according to the accompanying requisition. Determine which tubes are unlabeled, and credit the tests corresponding to the unlabeled tubes(s). Occasionally, one or more labeled tubes may be packaged with the unlabeled specimen, and these labeled tubes can be processed for testing. Place the small portion of the original barcode label on the unlabeled tube so that is can be Spec Tracked and retrieved if necessary. Place the large barcode label on the Reference Lab requisition with a comment stating that the specimen was unlabeled. This label alerts the client service billing employee to discharge a patient account number is no tests were performed. These specimens would be accessioned according to the name and tested as long as they could be paired with their original requisitions. If the client insists that the specimen be tested, a specimen release form is required via fax or courier from the client stating that they take responsibility for the results from an unlabeled specimen. Finally, all requests for testing on unlabeled specimens must be reviewed prior to testing by a supervisor or lead tech. These charge personnel must ascertain that the test requested is a screening test and not a diagnostic test. Page 2 of 2 Carolinas Laboratory Network Carolinas Medical Center Unlabeled Specimen Release Form I (please print name) request that Carolinas Medical Center Laboratory perform the following test(s) on an unlabeled specimen received from my facility. Critical values are those abnormal test results that could potentially be life threatening. Critical tests are those identified tests that require rapid communication of results, even if results are normal. All critical values should be called immediately and all critical tests must be called within timeframe noted on critical test list. Results are called by the reporting laboratory directly to a medical professional in the facility associated with the patient or client. If that medical professional is unavailable, you must then request the charge nurse of that location. See item #4 below for special instructions on microbiology critical values for the emergency department. During regular business hours of a client facility, notification attempts continue to occur until successful. In the event a physician does not respond to a page after hours, a second attempt occurs. If report cannot be given to her directly, the attending emergency medicine physician must be contacted. All Other Times Microbiology critical values must be called to an attending emergency medicine physician immediately. For pediatrics (ages 0-17), contact Pediatric Emergency Medicine Attending at 355-6580. Failed attempts for tests resulted in Sunquest should be documented as noted above and critical tests that are resulted in CoPath should be documented in the report. Results held to the following business day should include the date the result was called. This test code documentation is only visible to laboratory staff and will not appear on the patient record.